Biology notes and summaries
Biology notes and summaries
The following texts are the property of their respective authors and we thank them for giving us the opportunity to share for free to students, teachers and users of the Web their texts will used only for illustrative educational and scientific purposes only.
The information of medicine and health contained in the site are of a general nature and purpose which is purely informative and for this reason may not replace in any case, the council of a doctor or a qualified entity legally to the profession.
Biology notes and summaries
Biology Chapter 1 Notes
- The physical state of water depends on temperature, but its chemical composition is always the same. Living things are mostly water.
- Molecules are the smallest units into which water can be divided and still be water
- An electric current with proper conditions will break apart water into elements, substances that cannot be broken down chemically into simpler substances.
- Dalton made up the atomic theory, and named atoms, the smallest unit of an element. Molecules are made of atoms that have combined chemically, and it can be from more than one type of water.
- Elements are named by symbols, which start with a capital letter and can have another lowercase letter.
- Organisms are mostly made of water, but about 97% of the compounds are made of C, H, O, P, N, and S. These are essential.
- A subscript shows the number of atoms in each element.
- Atoms are made out of subatomic particles. Electrons are negative. Protons are positive. Neutrons are neutral.
- Protons and neutrons are in the nucleus of the atom. But the electrons form a negatively charged “cloud” around the nucleus since they’re attracted to the protons. They’re distributed throughout the cloud based on energy levels (or attraction) called electron shells. Ones nearer the nucleus are held more tightly.
- Hydrogen is the simplest atom. Since it only has one electron in its shell, however, it has room for another, which makes hydrogen very reactive.
- The first shell can hold two atoms, the second 8, the third 8. Atoms with filled shells are more stable.
- Atoms with unfilled shells have a strong tendency to lose/gain electrons to fill their shells.
- Atoms with differing numbers of neutrons are called isotopes. Radioisotopes have unstable atomic nuclei that break down and release radiation. They can help determine some of the chemical reactions organisms carry out.
- Chemical bonds are the attraction, sharing, or transfer of outer shell electrons from one atom to another. Ionic and covalent bonds form during the formation of a compound. Weak are formed due to the attraction between two compounds.
- Chemical reactions involve the making/breaking of chemical bonds. Substances interact and form new bonds and new substances.
- Only electrons in outer shells (valence) of the atoms are involved during reactions.
- Chemical reactions occur in cells, which are the basic units of life.
- Chemical reactions are important because they form new molecules that the cell needs for growth and maintenance. They also involve changes in energy (stored, used, or released).
- The law of conservation of matter states that matter can neither be created nor destroyed in chemical reactions. This means that a coefficient is added before some products or reactants to balance the equation.
- Activation energy is needed to get a chemical reaction started. For example, hydrogen and oxygen cannot combine without heat. Energy is released as light and heat.
- An ion is an atom or molecule that is positive or negative as a result of gaining or losing electrons. Cations - +. Anions - -. An ionic bond is the attraction between oppositely charged ions. In NaCl, an electron passes from sodium to chlorine.
- In a covalent bond, two atoms share one or more pairs of electrons. water, though, the oxygen atom attracts the electrons more strongly than hydrogen does. This gives water a slight negative charge and each hydrogen a slight positive charge and makes water polar. But if the electrons are shared equally, like in hydrogen gas, it’s called nonpolar.
- The polar nature of water makes it important. Molecules have to dissolve in water to move easily in and between cells. Polar molecules and ions dissolve because of the electric attraction between them. Nonpolar molecules like fat and oil don’t dissolve in water.
- Polar molecules can also form hydrogen bonds since there’s a slight charge. For example, a slightly negative atom of another molecule can be slightly attracted to a slightly positive atom of water.
- When NaCl dissolves in water, Na and Cl dissociate, but remain as ions in solution. Sodium’s important in regulating water balance.
- Ionization is when a nonionic compound is converted to ions. It separates into an H+ and an OH- ion. This happens to water every 1/500 million molecules.
- The pH scale is a logarithmic scale that runs from 0 – 14 that descries the level of H+ and OH- ions in solution. Pure water has 7 pH. Below 7 pH, things have more H+, so it’s more acidic. Higher than 7 pH, things have less H+, so it’s more basic/alkaline. A change of one pH unit = a tenfold change in the level of H+.
- pH of a cell’s interior helps regulate the cell’s chemical reactions. So organisms have ways to control it and respond to changes in the pH of their environment. Some bacteria and fungi can grow in acidic solutions.
- In Organic compounds, carbon atoms are combined with hydrogen and usually oxygen. They can also contain N, S, or P.
- Carbon atoms can combine in chains called carbon skeletons that form the backbone of macromolecules. Then other things can attach to it.
- The four macromolecules are: carbohydrates, lipids, proteins, and nucleic acids.
- Carbohydrates have a 2:1 ratio of hydrogen and oxygen atoms. They do not contain nitrogen.
- The simplest carbs are monosaccharides – glucose, fructose, and galactose. Glucose is used for energy. Biologically important sugars often have a phosphate group attached to the C-skeleton (sugar-phosphates).
- Two monosaccharides can bond to form a disaccharide – sucrose (glucose + fructose), lactose (glucose + galactose), or maltose (two glucose).
- Glucose can bond to form polysaccharides, like starch and cellulose in plants. Starch stores energy. Cellulose makes up the cell wall. Human liver and muscles store carbs in glycogen (animal starch). Polysaccharides have no fixed size.
- Lipids have two functions: long-term storage of energy and carbon, and building of structural parts of cell membranes. Lipids do not contain nitrogen – they contain C, H, and O.
- The monomers of fats are three fatty-acid molecules and one glycerol. They bond to form a triglyceride.
- Unsaturated fatty acids have double bonds and have “kinks” where the double bonds are. They’re oily liquids at room temperature. Saturated fats tend to be solids at room temperature. They’re more efficient for energy storage because they contain more hydrogen.
- Phospholipids form when glycerol combines with two fatty acids and a phosphate. The polar phosphate lets lipids associate with water. They form cell membranes with proteins. Cholesterol is part of the membrane structure of animal cells. They manufacture sex hormones.
- Amino acids make up proteins. Proteins are structural components as well as receptors between cells. They have structure, storage, hormone, antibodies, receptor, movement, enzyme, and transport. They’re also called polypeptides.
- Amino acids are made of COOH, amino, C, H, and R. There are 20 of them. When they covalently bond, it’s called a peptide bond. A long chain of amino acids is a polypeptide. Their sequence forms the primary structure (dictated by DNA) of a protein. The second structure is the primary structure folded because of H bonds – alpha helix and beta sheet. The Tertiary is the overfolding of secondary and there are many more bonds (globular, spherical). Quaternary structure is in hemoglobin and collagen and are made of 2+ polypeptide chains.
- One of the forces controlling how a protein folds is hydrophobicity (tendency for nonpolar amino acids to avoid water). So when it folds, nonpolar amino acids are inside folded proteins. The tertiary structure determines a protein’s function because of its shape.
- Proteins have no phosphorus.
- Nucleic acids dictate the amino-acid sequence and are the source of genetic information in chromosomes. They are the chemical link between generations. Nucleotides make up nucleic acids. They’re made of a phosphate, sugar, and a nitrogeneous base bonded covalently. The phosphate always remains the same. The sugar can be ribose or deoxyribose (which lacks one O). DNA is connected by the phosphate and the third carbon. The sugar for both RNA and DNA is 5 carbon pentose.
- Nitrogenous bases consist of: purines (adenine and guanine; double C rings) and pyrimidine (thymine, uracil, and cytosine; single C ring). DNA does not contain uracil; RNA does not contain thymine.
- Adenine complements thymine or uracil; cytosine complements guanine.
- One strand itself is bonded covalently, but when 2 attach together, they attach at nitrogenous bases. When adenine is attracted to thymine, there are 2 H bonds. When cytosine is attracted to guanine, there are 3 H bonds.
- DNA always occurs as a double-stranded helix. RNA is single-stranded. There are 3 types of RNA, which is made by DNA – messenger RNA (which assembles amino acids), transfer RNA (which transfer amino acids), and ribosomal RNA.
- Rosalind Franklin found the DNA Double Helix, but Watson and Crick explained it.
- DNA forms genes that pass from parent to offspring. DNA stores info in a code that has units made of 3 nucleotides (triplet codons). Some codons are translated by the cell to mean amino acids.
- Carbonic acid is a buffer that maintains the pH of a soslution.
Biology notes and summaries
Biology Chapter 2 Notes (Simplified)
- Living organisms: take in and convert materials and energy from the environment, release wastes, have a high degree of chemical and structural organization, contain DNA for maintaining their organization and activities, sense and react to changes in environment, grow and develop, reproduce, communicate, and move by themselves.
- Chemical energy is energy stored in organic molecules making up an organism. Free energy is available to do work. Energy can also cause change.
- Chemical work makes and decomposes polymers. Transport work moves monomers (nutrients) and increases cell organization.
- Heterotrophs obtain energy/nutrients from other organisms. Animals, fungi, and most bacteria are heterotrophs.
- Autotrophs obtain nutrients from nonliving things, like plants and some bacteria. They use the energy captured to synthesize organic compounds from inorganic things from their surroundings.
- Photoautotrophs use photosynthesis, where they capture energy from sunlight and use it to synthesize organic compounds from carbon dioxide and water. Some of the energy is stored. Chemoautotrophs use chemosynthesis to capture energy from inorganic chemicals in the environment.
- The organic compounds from autotrophs are building blocks for growth, maintenance, and reproduction.
- Heterotrophs consume autotrophs and other heterotrophs as food. Autotrophs directly or indirectly supply the energy and nutrients heterotrophs need.
- Cell respiration releases the free energy of organic compounds.
- Producers produce food (autotrophs). Consumers consume food (heterotrophs). Decomposers break down food and use them. They form a food web. The energy and nutrients flow from the producers à consumers à decomposers.
- Decomposers complete the breakdown of organic nutrients and return inorganic nutrients to the soil or water. Plants reabsorb these nutrients.
- Abiotic factors are nonliving, like soil and water. Biotic factors are living. They make up an ecosystem (forest, pond, etc.). In each ecosystem, there are many habitats, where certain organisms live. All the ecosystems make up the biosphere.
- The first law of thermodynamics says that the total energy is constant (it can change form). Energy is stored in molecules. When there’s a chemical reaction, some energy escapes as heat. Heat does not do anything except up the temperature. In living things, the conversion of chemical energy releases free energy to do work. Digestion breaks down polymers.
- The second law of themodynamics says that free energy decreases and entropy increases. This is because the energy spreads out into the surroundings and then the free energy in a system is less than before. This means that organisms should be well organized to live and grow.
- Energy maintains organization. Free energy comes from the environment. When energy is converted, some escapes as heat. There is only a one-way flow of energy through food webs à the end result is heat.
- More temperature increases enzymatic activity to a maximum point. Enzymes lower activation energy needed for a reaction to occur. They are also called catalysts. They are reusable.
- An enzyme has an active site on its tertiary structure. It is shaped closely to the reactant(s)/substrate(s). They have induced fit, which makes a substrate enzyme complex. This lowers the activation energy. Many reactions catalyzed by enzymes are reversible. Enzymes are specific to a temperature and pH.
- Metabolism consists of all the chemical activities of a cell. Synthesis makes polymers. Decomposition breaks down polymers. Decomposition gives free energy because it increases entropy. Biosynthesis uses energy.
- Oxidation removes electrons from a molecule. Free energy and heat is released and then follows a series of electron transfers. It ends up in ATP, adenosine triphosphate. The energy is in the bonds broken/made between its second and third phosphate. The third phosphate group then goes and attaches to something, which gives free energy to the something and activates it. The energy in ATP cannot be stored. It’s not stable; there is so much energy that the bonds are constantly breaking and releasing energy.
- When ATP gives up a phosphate, it becomes ADP. Then the ADP uses free energy and becomes ATP again. ATP is an energy carrier, a go-between for metabolism reactions, the “energy currency.” GTP and UTP are also like ATP.
- Energy is used to remove wastes, take in nutrients, maintain ion concentrations, and move from place to place.
- Food provides raw materials and cell energy. Digestion breaks down food.
- Physical digestion is breaking down large pieces of food into smaller ones, like chewing and grinding. This creates large surface area, and allows enzymes greater access to the food particles.
- Chemical digestion involves food breaking down into monomers. Enzymes move this along. This is how we absorb nutrients.
- Animals do extracellular digestion, which takes place outside the cell. They secrete digestive enzymes into a digestive cavity. Then chemical digestion occurs. Plants do intracellular digestion. Digestive enzymes break down the food. Some plants, like Venus flytraps, are special. They capture insects and digest them in special cavities in the leaves. Fungi digest materials from dead plants and animals. They digest food outside then absorb the nutrients inside.
- Complex multicellular animals digest food in cavities or digestive tubes with two openings, like an earthworm. Food goes into the mouth, and indigestible things go out through the anus. Goats and cows have four-chambered stomachs to digest. Horses and rabbits’ stomachs have special side pockets where microorganisms live and help digest cellulose. Since humans don’t have these pockets, they can’t digest cellulose. A carnivore’ digestive tract is shorter than a herbivore’s.
- Ingestion is the process of taking food into the digestive tract. It begins in the oral cavity. You chew and saliva is mixed. Saliva contains digestive enzymes, which begin chemical digestion. The food passes over the epiglottis, which prevents stuff from entering the trachea (airway). Then it goes to the esophagus, which connects the oral cavity to the stomach. Peristalsis, or wavelike contractions of the esophagus, move food to the stomach. Then it reaches the stomach. Muscles contract in the stomach and break up the food and mix it with gastric juices, made of enzymes, mucus, and acid. Then something relaxes and small amounts of food are emptied into the small intestine. Chemical digestion is completed here. Pancreas and liver contribute digestive juices. Then the blood carries the nutrients to the cells. Undigested material goes to the large intestine, where bacteria are. They help produce vitamins, gases, etc. Vitamins and water are also reabsorbed. This partly dries out the wastes (feces), which then go out through the anus.
- Carbohydrate digestion begins in the mouth with salivary amylase. They digest starch to shorter polysaccharides or maltose. Since the stomach has a very low pH, no carbohydrate digestion takes place in the stomach. It’s completed in the small intestine. Then the pancreas delivers pancreatic juices that convert the acidic food to a basic pH. The final result is glucose.
- Protein digestion happens in the stomach and small intestine. They need a strong acid environment, which is made by HCl in the stomach. The stomach walls are protected by mucus. When food enters the stomach, gastrin is made. Gastrin stimulates the secretion of HCl. The nervous system can also affect this. In the stomach, there’s pepsinogen, an inactive form of pepsin. HCl changes pepsinogen to pepsin. Pepsin breaks large proteins into small proteins. Then the small intestine makes them into amino monomers. Humans can only make half of the essential amino acids by themselves.
- When food enters the small intestine, pancreatic juice enters again and shifts the pH to basic. This lets intestinal enzymes function. Then trypsin breaks peptide bonds.
- Fats are also digested in the small intestine. They don’to mix with water. Enzymes only digest the fat molecules on the surface of the fat. They’re prepared for digestion by bile, which is secreted by the liver and stored in the gallbladder. Bile breaks down fat droplets, which increases surface area. Then lipase, which is secreted in the pancreatic and intestinal juices, splits fats into fatty acids and glycerol.
- Cells absorb the nutrients through their cell membranes. The molecules pass through the cells lining the small intestine, which is increased a lot by villi. Each villi contains capillaries. Then blood carries it to the cells.
Biology notes and summaries
Biology Chapter 3 Notes
- The simplest living organism is a single-celled bacterium. Its cytoplasm is surrounded by a wall (carbohydrates and proteins) and a membrane (phospholipids). They protect the cell from the outside environment, letting only useful things in.
- Useful things include water. Cells conduct many reactions that use soluble molecules. They need oxygen for releasing energy. They need the correct balance of ions. (Na, Mg, Ca, H, Cl, K). They need nutrients for energy and building material.
- Ammonium is potentially toxic. Autotrophs get carbon dioxide to build food.
- The polarity, size, and electric charge of molecules say whether they can pass through a membrane. The membrane lets in small hydrophobic molecules and small uncharged polar molecules, but turns away monomers and ions. The ones that can’t go in go in with the help of transport proteins.
- A membrane is selectively permeable (the membranes regulate the exchange of materials in a very specific way).
- Glycoproteins and glycolipids have sugars attached to them. They act as antennae that receive chemical messages.
- Diffusion is the movement of molecules from an area of higher concentration to an area of lower concentration; since there are more molecules in higher concentration, molecules move to where there is more space. This creates a concentration gradient, since there are differences in concentration of molecules.
- Entropy increases as diffusion occurs. When there’s equilibrium, there’s still moving.
- Since membranes are selectively permeable and don’t allow everything, concentration gradients can build across the cell membrane. This way potential energy is stored, since it’s like holding back the energy that could be running through. It’s based on the concentration gradient of substances. Ions form electric potential energy. This electric energy helps nerve impulses.
- Concentration gradients make energy for a lot of cell processes, like membrane transport.
- Osmosis is the movement of water down its concentration gradient and is also the movement of water across membranes. If an animal cell is placed in pure water, the water outside the cell is higher than inside, so water comes in. When it comes in, it can burst if it’s too much. But if there’s a concentrated solution outside, water goes out and hen it shrinks. The same goes for plant cells when they shrink, but when water goes into them, they don’t burst. Instead, they get more turgor, which makes them look healthier and stronger. This is because of the cell wall.
- The rate of diffusion depends on the size of the concentration gradient and the surface area of the membrane.
- Passive transport doesn’t need energy. Active transport needs energy because it moves stuff against their concentration gradient.
- Facilitated diffusion is where molecules move down their concentration gradient with the help of transport proteins. But it’s passive transport since it’s down the gradient. It speeds up the rate. It forms an open channel or attaches to and carries molecules.
- Active transport is fueled by the hydrolysis of ATP or from moving one cell against its concentration gradient against another thing going down its concentration gradient. Keeping up a certain gradient lets internal conditions keep up life. Enzymes need low Na, high K, and neutral pH. Plants need nitrate.
- Endocytosis takes in big things. Exocytosis moves big things out of the cell (waste or necessary).
- Cell respiration needs oxygen, and gives off carbon dioxide. Interior cells need nutrients, water, oxygen or carbon dioxide, and a way to remove wastes.
- Gas exchange occurs by diffusion across a membrane. The gases involved must be dissolved in water for diffusion to take place.
- Water contains little dissolved oxygen. So fish need a very efficient gas-exchange mechanism. Their gills, which are made of filaments, makes gas-exchange efficient because of a large surface area related to volume. The filaments consist of cells surrounding capillaries (short narrow blood vessels). As water passes over to gills, oxygen and carbon dioxide are exchanged between the blood in the capillaries and the water outside the filaments. Oxygen diffuses from the water into the blood down its concentration gradient, while carbon dioxide goes from the gills to the water.
- The concentration of oxygen is higher in air, so land organisms have adapted in order to not dry out. So they dissolve gases in water on the exchange membrane. They exchange gases on the interior. This protects the surface from evaporation and allows a large area for exchange.
- Grasshoppers are special. They use a system of small, branched air ducts to carry oxygen. There’s extensive branching of the air ducts into smaller and smaller tubes. The smallest tubes touch muscles and tissues.
- In a lot of land animals, lungs are the organs of gas exchange. They eliminate the one-way flow of oxygen that gills use. When the diaphragm contracts, you inhale. When the diaphragm expands, you exhale. The air moves through the same passage. The diffusion surfaces of the lungs are exposed to a mixture of oxygen rich and poor air, so the concentration difference isn’t that much.
- Nose hairs moisten, filter, and warm air. Then air travels to the lungs, where it ends up in alveoli, which consist of capillaries. Oxygen and carbon dioxide diffuse across the alveolar walls and the capillary walls. Large volume of gas can be exchanged with blood because of so many alveoli.
- Plants secrete cuticle, a water-repellent waxy substance. Humans also secrete waxy stuff. This helps prevent the plant from drying out.
- In insects, water loss is minimized by spiracles (openings into the trachea) which close when levels of carbon dioxide fall below a certain point. Plants have openings called stomates, which are surrounded by guard cells. When there’s water on the guard cells, they open the stomata, letting carbon dioxide out. This results in water loss, called transpiration.
- When there’s not enough water, the guard cells close.
- Paramecium use contractile vacuoles to squeeze excess water from the cell. The exchange of materials maintains homeostasis, the balanced and controlled conditions in the internal environment of an organism.
- Simple organisms like sponges and Hydra excrete waste through the external surface. In bigger organisms, not enough of the body touches the external environment, so all the cells can’t dispose of all their wastes. So there are special organs for excretion and maintaining water.
- In fish, they excrete carbon dioxide through the gills; in salt water fish, they also excrete salt.
- Metabolism produces stuff more toxic than waste gases, salts, and water. For example, example,proteins and nucleic acids lose nitrogen when they are converted to carbs or fats. Amino groups removed from amino acids become ammonia.
- Paramecium, though, excrete ammonia directly through membranes or body coverings.
- Ammonia is more toxic than urea, so it’s found in organisms that live where there’s a lot of water. Organs in drier environments usually excrete urea or uric acid.
- The nephrons are collected in the kidneys. The kidneys process waste products of metabolism. Blood cycles through them, and waste is removed.
- The urinary system is formed by the kidneys, blood, and the plumbing that carries fluid formed in the kidneys out of the body.
- Blood enters through the renal artery and leaves through the renal vein. Urine leaves through the ureter, which drains through the urinary bladder. Then the bladder is drained through the urethra. The cup of the nephron, the glomerular/Bowman’s capsule, The ball of capillaries inside it is the glomerulus. Collecting tubules from the nephrons eventually empty into the ureter.
- Nephrons filter, reabsorb, and secrete.
- Filtration occurs in the glomerulus, where the fluid blood is put into the glomerular capsule. The filtrate (materials that cross from the capillaries to the capsule) include the plasma (liquid blood), nitrogenous wastes, and amino acids.
- Reabsorption and secretion take place in the nephron’s tubule. Needed substances are absorbed and then returned to the blood. Cells of the tubule wall selectively remove substances that were left in the plasma after filtration or returned by reabsorption. Then they secrete these substances into the filtrate. Ex: K+.
- Aldosterone regulates excretion of sodium and potassium. It’s secreted by the adrenal gland, and it’s stimulated when sodium levels in the blood are too high. So potassium ions are released from the blood into the tubule near the collecting duct. This is an example of feedback regulation, where substances inhibit their own formation.
- When there’s not enough water, blood pressure drops. The hypothalamus senses this and stimulates the pituitary gland to release antidiuretic hormone, which causes cell membranes of collecting ducts more water permeable. Then water passes from the collecting duct to the blood.
- Kidneys can also remove salt in small amounts. When people have high salt concentrations, though, because their bodies excretes more water than taken in.
- Nephrons remove nitrogenous wastes from the blood (urea), regulate blood pressure and water-salt balance, conserve blood blucose, and excrete salt.
Biology notes and summaries
Biology Chapter 4 Notes
What Are Autotrophs?
All organisms need a source of energy and carbon compounds for making life-necessary compounds.
Photoautotrophs (plants, bacteria, and algae) depend on photosynthesis for carbon and energy.
In short, photosynthesis uses light to make carbon dioxide into sugar. Then enzymes convert these sugars into amino acids, etc.
Humans depend on photosynthesis for many things, even oil and coal.
Chemoautotrophs thrive in extreme environments, where there is not enough sunlight for photosynthesis. They live at the bottom of the ocean floor. For energy, they oxidize inorganic substances like Iron and sulfur.
Overview of Photosynthesis.
Light consists of a vibrating electromagnetic field. (Electromagnetic Spectrum)
The longer the light, the less energy it uses. Gamma, X rays, and ultra violet rays have the most energy while infrared, micro, and radio/radar/TV waves have the least energy. Visible light is in the middle of the spectrum.
Visible light has a range of energies as well, with purple having the most energy and red having the least energy. They cause small, reversible changes in the molecules that absorb them. However, higher energy waves can damage the things that haven’t adapted to them.
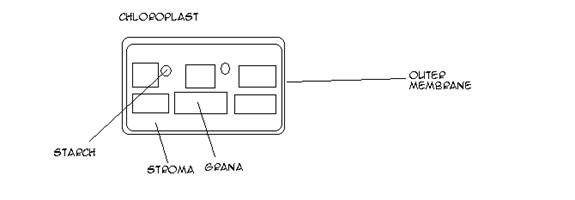
Pigments absorb visible light. In photoautotrophs, these are embedded in thylakoid membranes, which form closed sacs. The stacks of thylakoid membranes are called grana.
Thylakoids are part of a chloroplast, whose membrane separates the thylakoid from the cytoplasm of the cell (regulates flow of materials in/out of cell). Stroma (space) surrounds the thylakoids. It contains enzymes help catalyze phtotosynthesis. It also contains the chloroplast’s DNA, and the RNA and enzymes needed to make proteins encoded in the chloroplast DNA.
Chlorophyll is a pigment that reflects green light that is found in the thylakoids. There are two forms: a and b. Accessory pigments absorb more light and transfer it to chlorophyll a. In the fall, chlorophyll content drops and the accessory pigments are visible.
Instead of chlorophyll, some photosynthetic bacteria absorb light through a protein called rhodopsin (which is also found in animals’ eyes).
The three energy conversions of photosynthesis are: absorption of light energy, conversion of light energy into chemical energy, and storing the chemical energy in sugars, which occurs in the light reactions (absorb light à convert it to chemical energy) and the Calvin cycle (makes sugars from CO2).
Limiting factors like water, nutrients, light intensity, temperature, and the concentrations of carbon dioxide affect photosynthesis.
The light saturation point is the peak of photosynthesis before it declines when there is full sunlight. At light greater than the light saturation point, chlorophyll accumulates energy faster than it can transfer the energy to the electron transport system. Then the energy passes to oxygen, which can react with water to form hydroxyl or hydrogen peroxide, which can damage chloroplasts (decline in photosynthesis – photoinhibition).
Young pine trees need more like than young broad-leaved trees.
C3 plants grow best at temperatures between 20 – 30 degrees Celcius.
An increase in carbon dioxide concentration increases the rate of photosynthesis to a max point, after which the rate declines. Above the carbon dioxide saturation point, increases have no effect on photosynthesis.
Both oxygen and carbon dioxide are held by double bonds that keep the atoms the same distance apart, allowing rubisco to bind to either of them. When rubisco binds with oxygen, one PGA and one glycolate forms, which is transported out of the chloroplast and partly broken down to carbon dioxide (photorespiration – loses fixed carbon atoms)
Photorespiration slows photosynthesis down because of the loss of carbon. Higher levels of carbon dioxide favor photosynthesis, while higher levels of oxygen favor photorespiration. Weather also affects this: hot, dry weather causes stomates to close, not letting any gases in.
C4 plants include sugarcane, corn, and crabgrass. C4 plants have 2 systems of carbon dioxide fixation. They fix carbon dioxide to a 3-carbon acid, and the resulting acid is rearranged and then transported to the bundle sheaths, which surround each vein in the leaves. Mesophyll surrounds the bundle sheaths. Then carbon dioxide is released from the 4-carbon acid in the bundle sheath and refixed by rubisco, which forms PGA in the Calvin Cycle.
Advantages: Carbon dioxide is delivered efficiently to the bundle sheaths, and the concentration of CO2 in the bundle-sheaths favors photosynthesis This means that C4 plants can function efficiently at high temperatures while keeping stomates partly closed to reduce water loss.
C4 plants are about twice as efficient as C3 plants in converting light energy to sugars. Photorespiration may be a major factor limiting plant growth and world food production today (crops like soybeans, wheat, and rice are C3 plants).
CAM plants open their stomates at night and incorporate carbon dioxide into organic acids, which are broken down by enzymes to release carbon dioxide. However, these plants usually grow slowly even though they live in intense heat.
Photosynthesis and the Atmosphere
Most organisms use oxygen and release Co2 (including plants. Photoautotrophs use the Co2 again in photosynthesis.
Photosynthesis is the larges single biochemical process on earth. CO2 content of the atmosphere has been increasing steadily since 1800 (due to burning of fossil fuels or clearing land by burning rain-forest plants). This increase may have heated up Earth’s climates. Balance between O2 and CO2 must be preserved.
Early in the history of life there was little oxygen in the air. C4 and CAM plants are adaptation of photosynthesis to an oxygen rich atmosphere; however, since the 1980s there has been increased growth of C3 plants (the increasing CO2 in the atmosphere favors C3 plants).
Diagram Notes
- The upper geyser basin at Yellowstone – the water is too hot to support photoautotrophs, but the place is colorful due to chemoautotrophic bacteria.
- Photoheterotrophs include some bacteria. Chemoheterotrophs include animals, fungi, some bacteria and other one-celled organisms.
- Energy radiating from the sun forms a continuous series of waves (spectrum). Visible light, which animals can see, is roughly the same range that plants use in photosynthesis.
- Photosynthetic pigments are embedded in the thylakoid membranes. DNA, RNA, and Calvin cycle enzymes are in the stroma.
- Chlrophyll b differs from a in having a CHO- group in place of a CH3 group. Other forms of chlorophyll occur in algae and photosynthetic bacteria. The part with the magnesium as the central atom absorbs light. The hydrophobic tail helps to keep the molecule anchored in the lipid-rich thylakoid membrane.
- Chlorophyll a absorbs Blue and Orange light the most. Chlorophyll b absorbs Blue light the most. Photosynthesis absorbs purple, blue, and orange the most.
- Each photosystem consists of several hundred molecules of chlorophyll and accessory pigments that absorb light energy and transfer it to a special chlorophyll a molecule. The reaction center is the only pigment molecule that can participate directly in electron flow in the light reactions.
- Chromatium oxidizes hydrogen sulfide gas instead of water.
- Halobacterium is a photosynthestic bacterium that relies on bacteriorhodopsin to carry out photosynthesis. It is fairly small and its structure/action is similar to rhodopsin. It has seven sections that spiral across the bacterial cell membrane, which cluster together to form a channel through the membrane. Exterior channel amino acids have hydrophobic chains (associate with membrane lipids), while ones on the inside have hydrophilic side chains. Retinal (related to Vit. A)is weakly bound to one of the amino-acid side chains near the opening to the inerior of the cell (inside). When it absorbs light, it detaches from the protein and binds to an H+. Then it binds to another amino acid at the outer end of the channel (releasing H+); Cycle repeats, and light powers the transport of H+ out the bacterial membrane; then ATP synthetase uses the concentration gradient to power ATP production.
- Enzymes in the stroma catalyze CO2 fixation, requiring ATP and NADPH. ADP and NADP return to the light reactions. Enzymes catalyze the recominbation of five PGAL to form 3 RuBP.
- Plants use the sugars produced in photosynthesis to supply energy and carbon skeletons for growth and other cell work.
- Pathways and cycles are made of multiple copies of the enzymes and other molecules (enzymes may be attached); in the light reactions, enzymes and electron carriers are embedded in sequence in thylakoids, while Calvin-cycle enzymes in the stroma may also be attached to the chloroplast membranes. They are arranged.
- Vitalism – the theory that a life force makes living matter different from nonliving matter (Aristotle). In the Middle Ages-Renaissance, humans were thought to be made of fire, earth, air, and water. Animals were at higher levels on the same Great Chain of Being. 1700s – chemists (materialists) say that organism and their products are made of the same elements of nonliving things). Materialism – explain life by completely reducing it to chemical substances (complicated -> simple) (reductionist theory)
- 1800s – monomers identified; Loeb – salts force unfertilized sea urchin eggs to start developing; Miller passes electricity through gases and sees that the reaction produced amino acids. the common link among all life is a paritular chemical molecule. Consciousness is an emergent property because it emerges from a complex brain composed of many simpler parts.
- Plants do well at good light and at 25 degrees Celsius.
- Crassula is an example of a CAM plant.
- Biotechnology – create more productive food crops. Produce C3 plants where oxygen does not inhibit rubisco. First: change gene of rubisco so that the enzyme would not bind oxygen. Hopeless. Try to incorporate genes for the C4 system into C3 plants.
- Energy flows from the sun to organisms in one direction, but substances like oxygen and carbon dioxide cycle repeatedly.
Class Notes
Plants give us oxygen and nutrients.
Electromagnetic Spectrum
- Colors you see are the ones reflected to eyes (rest absorbed)
- White = all colors reflected
- Black = all colors absorbed
Photosynthesis: (“Light Creation”) Redox (Reduction/Oxidation)
Cross Section of a Leaf
Chloroplast (double membrane) in every plant cell; has own DNA/RNA
- Thylakoid membrane makes pouches.
- van Helmont said that water gives weight. (WRONG)
- Priestly said carbon dioxide gives plant weight.
- De Saussure says that light was important.
- Robert van Mayer said that plants convert light energy to chemical compounds.
- Carbon dioxide reduced to glucose (weight)
- Water oxidized to oxygen
Chlorophyll – pigment that reflects green light; central element Mg
- Light moves in as photons (discrete bundles of energy) and waves
- Photons excite the electrons in chlorophyll (PSI – chlorophyll A; PSII – chlorophyll B)
- Excited electrons leave the orbit and are accepted by the Electron Transport System (which is embedded on the thylakoid membrane) (ETS à PSI à NADP+ is reduced and H+ ions are added à NADPH)
- Light energy on PSII also splits water into oxygen gas which leaves cell through stomata. (Only in PSII does this reaction take place!); The electrons that are made from splitting water fill the hole in chlorophyll.
- Excited electrons pass through the ETS and go to Reaction Center PSI, which passes it to the electron carrier.
- H+ ions are too many in the stroma (If too many H+ ions, turn acidic). They are kicked by transport protein into the thylakoid space. Only ATP Synthase enzyme will let them diffuse back, which creates concentration gradient (let in, kick back out)
- This concentration gradient creates proton motive force, which gives enough energy on ATP Synthase
Light reactions
- Oxygen gas is a waste (No use in photosynthesis, though it’s used for cellular respiration
- NADPH and ATP are made. However, these are unstable and cannot be stored, and so must be used for the Calvin Cycle. This allows the Calvin cycle to reduce carbon dioxide into PGAL (simple glucose)
The Calvin Cycle
Photorespiration occurs during photosynthesis.
- When carbon dioxide concentration is low in a leaf, oxygen fixes itself with the help of rubisco (enzyme) in Calvin cycle with RuBP (C-5 compound) and all the RuBP is used up without producing PGAL.
CAM – very slow growth
- C. Acid Metabolism
- Juicy – cactus
- Stomata only open during night
- They take CO2 in night and convert it into oxacicetic of cell (C-4 compound) – organic acid (Bring in CO2, change, change back)
C4 plants – CO2 all the time because of fixing
- To overcome photorespiration, C4 plants also close their stomata during very hot period and fix CO2 when stomata are open during night or day CO2 fixed into organic acid (C-4 compound); grow faster than C-3 plants
Biology notes and summaries
Biology Chapter 5 Book Notes
- Metabolism consists of the chemical reactions in an organism. It has 2 parts: synthesis (monomers into polymers, consuming energy) and decomposition (polymers to monomers, releasing energy). Biosynthesis sometimes use carbon skeletons made in decomposition and use ATP, made during decomposition.
- Cell respiration is a stepwise decomposition pathway that provides energy by oxidizing sugars or other organic substrates. It has two types: aerobic (with oxygen, which is the oxidizing agent) and anaerobic (without oxygen, where the substrate may only be partially decomposed or have N or S replace O).
- The raw materials for aerobic respiration are carbs, fats, and proteins. Glucose (main form transported through blood in animals) and glucose-phosphate produce them by digesting carbs or by breaking down glycogen in the liver or muscles. This is matched by sucrose and starch in plants.
- Energy released as glucose gradually oxidizes and becomes carbon dioxide.
- Since oxidizing one glucose releases a lot more energy than is needed, a series of reactions occurs.
- Cell respiration provides ATP and carbon synthesis for biosynthesis.
- There are three main stages in aerobic respiration: glycolysis à (intermediate step) à Krebs cycle à electron transport system
- Since oxygen must accept the electrons at the end of the electron transport system, the Krebs cycle and the electron transport system cannot proceed without oxygen.
- Both aerobic and anaerobic respiration begin with glycolysis (where glucose is the raw material). ATP gives a phosphate to glucose to make it into glucose-6-phosphate, which is reaaranged (enzyme), and is given another phosphate (ATP). This lets the molecule split into 2 e-carbon sugar-phosphates, which then forms into pyruvate. Pyruvate is oxidized and NAD is reduced (while 4 ATP forms from energy released). Products: 2 NADH, 2 Pyruvate, 2 ATP (net)
- In glycolysis in plants, starch and sucrose decomposes to glucose or glucose-1-phosphate, which enter glycolysis immediately. Then 3-carbon sugar-phosphates formed in photosynthesis can enter.
- The roles of glycolyssi are the synthesis of ATP, NADH, and pyruvate and the formation of C-skeletons (from sugars/organic acids)
- If oxygen is not present, fermentation will happen. In animals, this is lactic-acid fermentation. Bacteria converts NADH and pyruvate into NAD+ and lactate (C3). NAD+ goes back to glycolysis, which continues on to provide 2 ATP. Examples of fermentation: yeast (pyruvate à ethanol) and bacteria (pyruvate à vinegar)
- Glycolysis and fermentation occur in the cytoplasm. The Krebs cycle and the electron transport system occur in the mitochondria.
- In bacteria, the electron transport system is embedded in a membrane, and all the enzymes of cell respiration are suspended in its cytoplasm. The enzymes of these pathways are synthesized according to the cell’s DNA coding.
- Mitochondria are called “cell powerhouses” because that’s where most ATP is synthesized. They provide efficiency and organization. Electron microscopes reveal their detailed structure. Mitochondria are bimembrane. The inner membrane is made of cristae (folds), where the enzymes of ATP formation and some for the Krebs cycle are located (Most of the enzymes for the Krebs cycle are in the fluid matrix of the mitochondria), while the outer membrane regulates materials in/out of the mitochondrion.
- In the intermediate step and Krebs cycle, the 2 pyruvates are oxidized, reducing NAD+ and FAD, and some ATP forms. Glucose is oxidized to carbon dioxide (gas).
- In the intermediate step, enzymes release a molecule of carbon dioxide from each pyruvate, making the two C3s into two C2s (acetates). 2 NAD+ are also reduced.
- Coenzyme A binds to the acetate (acetyl CoA) and enters the Krebs Cycle. An enzyme combines acetyl CoA with a C4 acid (oxaloacetate) to form citrate (C6). Two of the Cs in the C6 compound oxidize to Carbon dioxide (making it C4 and also reducing 2 NAD+ to 2 NADH). The C4 organic acid is rearranged and oxidized some more, which produces another oxaloacetate that starts another Krebs cycle.
- While this is occurring, the C4 compound reacts some more, reducing another molecule of NAD+ and one of FAD+, and some ATP forms.
- The products of the Krebs cycle are: 6 CO2, 8 NADH, 2 FADH2, and 2 ATP
- The electron transport system is made of easily oxidized and reduced enzymes/proteins called cytochromes, which are embedded in the inner membranes of mitochondria. They transfer the electrons throughout the system, which separated hydrogen into electrons and protons. The terminal (last) cytochrome) combines electrons with oxygen to form water. This is the only step of respiration that uses oxygen.
- Whenever the electrons are transferred, energy is released. Some o f this lets enzymes in the inner mitochondria actively transport protons from the matrix to the intermembrane space. Then this creates a high concentration of H+ there, so the protons diffuse back to the matrix of the mitochondria through the ATTP synthase. This continues on and on, which creates a concentration gradient that provides enough energy for the synthesizing of ATP from ADP and a phosphate. NADH can provide energy for up to 3 ATP, while FADH2 can do the same for 2.
- In bacteria, there are no mitochondria, and their cell membranes contain electron transport systems. Electrons flow to oxidizers other than oxygen, like sulfate or nitrate, and so instead of forming water, H2S or NH3 (ammonia) is formed. (Anaerobic respiration)
- Facultative aerobes (some bacteria) switch back and forth between fermentation and aerobic respiration (based on oxygen content). Obligate anaerobes generate ATP entirely from fermentation/anaerobic respiration (bacteria poisoned by oxygen). Most organisms, though, are obligate aerobes (cannot survive without oxygen).
- Animals have specialized organs and systems (lungs/circulatory systems) that deliver oxygen to the electron transport system, which helps release energy better.
- The products of photosynthesis (from photoautotrophs) are the raw material for cell respiration, which then provides the raw materials for photosynthesis. They both provide C-skeletons used in biosynthesis and use e- transport systems to form ATP.
- The release of energy from fats and proteins also involves the Krebs cycle. When cells use fatty acids for energy, mitochondrial enzymes break down the fatty acids to acetate. CoA then transfers this to the Krebs cycle, which decomposes it; however, since glycolysis did not occur, fats cannot ferment, so if there is no available oxygen, most of the energy in fat will not become ATP. In proteins, digestion decomposes them to amino acids. Then the amino groups are removed and converted to safer N compounds (from NH3), which are recycled or excreted. The C-skeletons from the AA can undergo reactions for form C4 or C5 compounds that enter the Krebs cycle.
- The Krebs cycle and glycolysis provide building blocks for biosynthesis. In autotrophs, they (with the Calvin cycle) lead to the synthesis of every organic compound needed, while in heterotrophs it leads to the synthesis of MOST of the organic compounds needed (others are eaten).
- Most synthesis pathways are not the opposite of decomposition. There are separate enzymes and pathways. For example, AA are synthesized into proteins precisely, while digestion is not so precise (keep breaking bonds until the protein is completely decomposed). This is an example of hydrolysis (breaking through water), which most biological decompositions involve. Specialized cells that line the intestines actively transport nutrients into blood.
- Cell respiration also releases heat, which is maintained by homeostatic mechanisms. Some mammals (hair and milk glands) have brown fat (tissue), which contains the most mitochondria out of all the body tissues; it produces thermal energy quickly. Respiration of stored fat there produces a lot of heat, but little ATP. It is located on the neck and between the shoulders, and is especially active at the end of hibernation.
- The mitochondria of many plants contain another branch of the e- transport system, where some of the energy of e- flow results in more heat and less ATP. For example, in one plant, organic compounds evaporate, producing an odor that attracts flies and beetles, which land on the flower. Pollen sticks to them, and when the bugs fly from plant to plant, the pollen fertilizes other plant. (Skunk cabbage – foul gases and melts snow).
- The storage of glucose in starch/fat or the decomposition of glucose is determined from supply and demand. Example: When energy is used rapidly, cells absorb glucose to make ATP. Liver then breaks down their stored glycogen to restore the blood sugar level. When energy is not used as much, cells use glucose to synthesize glycogen then fat.
Class Notes
Cellular Respiration
- oxidation (decomposition of digested food (nutrients)) that blood transports to every cell of all organisms.
Five Kingdoms
- Animalia
- Planti
- Fungi
- Protista
- Monera (Bacteria)
- Glycolysis – first step of Aerobic and anaerobic respiration
- Takes place in cytoplasm
- Partial breakdown of glucose into 2 pyruvate, 2 ATP, and 2 NADH
- If oxygen is available, pyruvate will be shuttled (using ATP) into mitochondria. If oxygen is not there, undergo fermentation.
- Fermentation takes place in the cytoplasm.
Steps of Fermentation
- glycolysis
- carbon dioxide and alcohol or lactic acid
Intermediate Step: C3 loses one C
Metabolism – all the chemical reactions in a cell
- Synthesis
- Formation of Molecules
- Needs energy
- Needs C-skeleton (photosynthesis)
- Decomposition
- Breakdown
- Releases energy
- Produces C-skeleton (simple compounds) (through Krebs cycle)
- Provid something which can make C-skeletons
- Plants have both mitochondria and chloroplast
- The smaller the animal, the more cell respiration needed (body heat maintenance)
Bacteria
- obligate anaerobe – cannot live in presence of oxygen
- facultative aerobe – can live with or without oxygen (Fungi also here)
obligate aerobe – need oxygen (most plants / animals)
Glycolysis - 2 NADH (à ETS), 2 ATP
Intermediate Step – 2 NADH (2 C3), 2 CO2 (waste)
Krebs Cycle (x2) – 6 NADH, 2 CO2, 1 FADH2, 1 ATP
ETS – 10 NADH, 2 FADH2
10 x 3 ATP = 30 ATP
2 x 2 ATP = 4 ATP
+ 4 ATP from Glycolysis and Krebs Cycle
= 38 ATP – 2 used in shuttling pyruvate to mitochondria
= 36 ATP
- Excess glucose stored in liver
- Adrenaline stimulate glucagons.
- All nutrients can be used for Krebs cycle, but in proteins N is removed and phosphate is attached.
Diagram Notes
- ATP mediates the transfer of energy in metabolism.
- Cellular respiration breaks down sugars and other organic compounds. The products include C-skeletons (biosynthesis) and ATP (provides energy for things like nerve impulses (animals), movement, active transport, and maintenance/organization)
- NADH acts as a carrier of reducing power. It is reduced when it receives e- and protons from glucose. It’s oxidized when it donates e- and protons to the electron transport system.
- Many of glycolysis’s steps involve rearrangements of carbon skeletons
- The fermentation of wine is by yeast. It releases energy from sugars without oxygen. Yeast produces ethanol and carbon dioxide. In the 1850s, Louis Pasteur found that bacteria contaminating the wine were fermenting it (they produced vinegar instead of ethyl alcohol).
- If there is no oxygen after glycolysis, then pyruvate undergoes fermentation, forming incompletely oxidized compounds.
- Energy released by the electrons as they pass through the ETS powers the active transport of protons out through the inner mitochondrial membrane, which diffuse back to the matrix through the ATP synthase.
- ATP forms as the electrons in NADH and FADH2 lose energy in reducing oxygen. FAD+ and NAD+ are recycled.
- The rate at which a polymer breaks down depends on the concentration and activity of the enzyme that decomposes it. Epinephrine (adrenaline) binds to proteins in the liver cell membranes, stimulating kinase to add phosphate group to other proteins. Another kinase stimulates the enzyme to break down glycogen. Enzyme cascade. Oxygen starved muscles begin to ferment glucose to lactate, causing pain and cramps. Blood caries the acid out of the muscles and back to the liver, to be converted back to glucose.
- Particularly the Krebs cycle contribute to the decomposition/ biosynthesis of carbs, fats, and proteins. Certain amino acids can be synthesized from C-skeletons by removing amino groups. Most organisms cannot convert fat to carbohydrate.
- When oxygen is there, cell respiration converts lots of ADP to ATP, inhibiting PFK (slowing down glycolysis). When oxygen is not there, ADP builds up, stimulating PFK. This is negative feedback, where the product of a process inhibits its own synthesis.
- Whenever blood sugar rises, insulin is produced, which binds to insulin receptors (cell surfaces), which tells the cells to absorb more glucose and convert it to glycogen or fat. Diabetes is when there is not enough insulin or its receptors. When blood sugar falls too much, glucagons makes cells break down glycogen to glucose and release it in the blood.
- The arctic ground squirrel spends its summer on the Arctic tundra, but hibernates in the winter. Brown fat lets it elevate its body temperature. Skunk cabbage releases heat energy that melts snow when it’s emerging.
- Hydrolysis: Bonds are broken in a water molecule and another molecule. The H_ and OH- formed from water bond to the fragments of the polymer. This breaks down fats, polysaccharides, and proteins.
- Supply and demand determine how glucose and fatty acids are used. The rates of biosynthesis and decomposition vary because of energy demand. When there’s low energy demand, it is stored in starch and glycogen or fats. When there’s high energy demand, the starch, glycogen, and fats, are broken to sucrose, PGAL, and glucose. Fat cells produce leptin and release it to the blood, which binds to a receptor protein on brain cells. This says that the stomach is full. It also lets cell respiration increase and reduce formation of storage lipids. Obese organisms have been found to have less leptin. However, high leptin levels block the response of liver cells to insulin, making people diabetic. It also reduces mineral density of bone.
Biology notes and summaries
Biology Chapter 6 Notes
Cell Study and Technology
- The cell is the basic unit of life.
- The cell theory states that cells, which come from preexisting cells, or their products are the units of structure and function in organisms.
- Progress in studying cells depended on improved microscopes, better techniques preparing cells for observation, and cell function studies.
- Electron microscopes have a magnification of more than a million; however, samples are killed before they can be observed, or the sample preparation can alter cell structures. Scanning tunneling microscopes can be more powerful and do not need harsh treatment to samples, but it can only reveal surface features.
- Cells average about 10-20 um in diameter, but bacteria can be only 1 um long.
- The human adult is about 1 um, a fish egg is about 1 mm, a bacteria is about 1 um, and the thickness of DNA is about 1 nm.
The Cell Theory
- Robert Hooke examined cork, saw little boxes, and called the compartments cells.
- Robert Brown saw a dense object in cells (nucleus).
- MJ Schleiden said that plants are made of cells that contain nuclei and cell fluid.
- Theodore Schwann said that animals are also made of cells.
- Anton van Leeuwenhoek observed microorganisms in water.
- Eventually it was found that microorganisms were single-celled. Cells were seen to produce more cells.
- Rudolf Virchow said that: “All cells come from cells.”
Two Basic Types of Cells
- Living cells are separated into prokaryotes and eukaryotes.
- Prokaryotes are bacteria and are simple. They are everywhere and nearly always unicellular. Some can be 0.3 um in diameter (though the average size is 1-5 um). They lack mitochondria, chloroplasts, and a nucleus.
- Eukaryotes are larger (10-50 um) and more complex. They form multicellular organisms: plants, animals, and fungi. They have many parts with specific functions; this gives them flexibility. They have mitochondria and chloroplasts. They contain a nucleus, which contains the DNA of eukaryotic cells; this DNA directs the synthesis of enzymes and proteins that direct everything in a cell.
Prokaryotic Cell Structure
- Prokaryotic cells have a cell wall made of peptidoglycen, but do not contain cellulose. They have one chromosome made of a continuous circular molecule of DNA, which is attached to the nuclear region (nucleoid). Also, they contain 1+ smaller circular DNA molecules (plasmid), which contain most of the cell’s DNA and are attached to the plasma membrane. The part of the plasma membrane attached to the chromosome may contain enzymes that can help replicate the chromosome before the cell divides in two.
- The three common shapes of bacteria are rod (bacilli), spheres (cocci), or corkscrews (spirochetes). Some also have flagella, an extension of the cell membrane made of protein that lets cells swim through water or body fluids. The ones with flagella can sense substances.
- Bacterial decomposers help recycle nutrients like C, N, and S. These reactions also provide prokaryotes with free energy or fixed C and N. They also recycle nutrients that would remain unavailable in wastes and dead stuff. A lot of prokaryotes are autotrophs. They are primary producers in lakes and oceans. They are also useful as the source of antibiotics and things hard to make artificially. Bacteria in the large intestine provide vitamins and help digest food. They also help ferment food like cheese and yogurt and drugs.
Eukaryotic Cell Structure
- Eukaryotes are divided into organelles, unlike prokaryotes. Each part has its own structure and function. A lot of chemical reactions occur at the same time because the membrane divides the cell into compartments. Reactions may produce something in one place and use it in another. This enables the cell to separate specific processes and have division of labor.
- Both eukaryotes and prokaryotes’ contents are enclosed by a plasma membrane. The plasma membrane of plant and fungal cells (as well as some unicellular eukaryotes) are surrounded by a rigid cell wall, made of stiff fibers of cellulose and carbs. Animal cell’s don’t have a cell wall.
- The nucleus is the cell’s genetic control center (chromosomes). A bilayer of membranes forms the nuclear envelope / membrane surrounding the chromosomes. The chromosome consists of a long DNA molecule wrapped around protein spools. 1+ drops of concentrated RNA are usually visible in the nucleus, called nucleoli, which are the sites where types of RNA that will help synthesize protein are made.
- The stuff surrounding the organelles and the organelles is the cytoplasm. The organelles float in the cytosol, which an organized gel where components have specific places. The cytoskeleton s a network of protein fibers that helps shape and organize the cell. It is made of microtubules, microfilaments, and connecting intermediate filaments. It can hold organelles in place or move them around. Organelles are loosely bound to the cytoskeleton or to other proteins/solutes.
- Ribosomes (in both types of cells) are made of RNA and protein. They catalyze the synthesis of proteins. They are found on the rough endoplasmic reticulum. The endoplasmic reticulum is a system of membranes that connects many of the organelles in the cell. Proteins made by ribosomes attached to the ER go into the ER when they’re formed, then are transported to where they need to be (though they may be modified in the Golgi apparatus). These proteins can become part of the cell membrane or end up inside organelles. It also carries other substances.
- The Golgi apparatus is a series of membranous sacs; as material passes through it, it is packaged in spherical, membrane-enclosed vesicle, which can fuse with the plasma membrane (releasing their contents outside the cell) or deliver their contents to other organelles.
- The ER, Golgi apparatus, and vesicles make up a connected internal membrane system, which lets it act as a highway that directs proteins.
- Lysosomes are special vesicles in animals and other eukaryotes. They have enzymes that decompose old macromolecules for recycling. They can also fuse with vesicles made by endocytosis (digesting food). Some lysosomes in animals fuse with the plasma membrane. Then digestive enzymes are released outside the cell, breaking down bacteria.
- Vacuoles are in plants. They are vesicles that enlarge as cells mature and contain water, organic acids, digestive enzymes, salts, and pigments.
- Chloroplasts and mitochondria are bimembrane organelles involved in energy reactions. Photosynthesis are in plants and photoautotrophic eukaryotes. Mitochondria are major sites of ATP synthesis.
- Centrioles are tubular structures in the cells of animals and some fungi/algae. They participate in cell reproduction there. They are made of a pair of cylindrical bundles of microtubles.
- Eukaryotic flagella are long bundles of microtubules. Enzymes associated with these provide energy for the motion of the flagellum.
- Cilia are short flagella. They cover cells in rows. They move cells along and help move material along a cell or tissue.
Cooperation Among Cells
- When one-celled organisms divide, some new ones cluster together. They live in groups called colonies. Members are usually related. Bacteria that attach to solid objects form biofilms, which can contain bacteria that require similar environments. They cooperate to change conditions to suit their own needs.
- Individual cells take on specialized roles, like Volvox forming a colony in the shape of a hollow ball.
Biology notes and summaries
Biology Chapter 8 Notes
Prokaryotes (yeast, amoeba, etc.) divide in two – binary fission. Eukaryotes’ cells also reproduce by dividing in two through stages called the cell cycle.
By dividing into many cells, an organism’s surface area can keep up with its volume.
Plants have specialized regions at the tips of their roots and stems, where cells divide to produce cells that develop into the tissues of roots, stems, leaves, etc.
Animal cell division produces cells that form the nerves, skin, etc.
The timing of cell division is critical to development – cells in developing tissues pass through the cell cycle at different rates and coordinate their development with neighboring cells to produce organ (systems).
Eukaryotic cell division needs accurate replication and equal division of the genetic information in the cell’s DNA. Each daughter cell must inherit an identical set of chromosomes.
In humans, an error in DNA replication/cell division can lead to birth defects, cancer, etc.
Cell division replaces worn out/dead/damaged cells, help us to grow, and heal.
The cell cycle is remarkably similar in all eukaryotes.
When a cell completes the cycle, it becomes two new daughter cells.
The Phases of the Cell Cycle
In mitosis, a cell’s nuclear membrane breaks down, and its chromosomes separate and become visible as they are distributed to the daughter cells.
Interphase is the period between divisions and it consists of Gap1, S, and Gap2. Chromosomes are not visible.
During G1 and G2, cells grow and synthesize RNA, proteins, and other polymers. G1 is before S and is where the cell enlarges and makes new proteins. G2 is before mitosis and is where the cell prepares to divide. In S, cell replicates DNA.
G0 is a stopping point within G1, where most cells in adult multicellular organisms are. They are metabolically active and specialized to perform life-sustaining tasks.
Some signals reach the cell and stimulate cells in G0 or G1 to pass through the restriction point, where it will go on to divide (complete the cell cycle).
Stem cells in bone marrow constantly divide to produce replacements for worn-out red blood cells. Liver cells normally remain in G0 unless it’s removed in surgery; then cells divide until the liver reaches its old size.
Mature nerve cells (like brain and spinal cord) and muscle cells rarely divide.
Cells go from the restriction point to the S phase. Here, the DNA of each chromosome replicates, forming new identical set of chromosomes and doubling the number of genes in the nucleus.
From S phase, the cells go to G2. Here, RNA and proteins are synthesized which are required for mitosis.
During interphase, the chromosomes spread out and fill up the nucleus.
In mitosis, also called nulceur division, the nucleus divides into two nuclei with identical sets of chromosomes. It ensures that each new daughter cell receives one copy of each chromosome.
From mitosis, the cell goes to cytokinesis, when it completely divides.
DNA replication depends on the molecular shapes of DNA and its nucleotide bases, which pair depending on how many hydrogen bonds each nitrogen base can form with its counterpart.
Adenine has two hydrogen bonds when attracted to thymine.
Cytosine has three hydrogen bonds when attracted to guanine.
The sugar-phosphate backbones of the two strands have opposite orientations but parallel (antiparallel).
DNA synthesis in the S phase has 3 major parts: binding of enzymes to existing DNA, unwinding of the double helix, and synthesis of a new matching strand for each existing strand.
First, enzymes/proteins in DNA synthesis bind to replication origins on chromosomes. The proteins include an enzyme that unwinds the DNA double helix, an RNA-synthesizing enzyme, and DNA polymerase, which catalyzes the formation of the DNA strands.
The combination of DNA and proteins is called a replisome.
In prokaryotes, there is only one origin of replication since it has only one chromosome. Eukaryotes have many more chromosomes, so they have more replication origins.
DNA polymerase can add nucleotides only to the end of an existing nucleic-acid strange (phosphate, sugar, nitrogenous base). It synthesizes a short matching section of RNA that acts as a primer for DNA synthesis. The base sequence of the existing strand determines the sequence of the matching strand (complementary bases)
Synthesis of the new matching strange occurs continuously on the leading strand (one of the original). Here, DNA polymerase ads DNA nucleotides to the end of the RNA primer. The replisome unwinds the DNA as it moves away from the replication origin. DNA polymerase extends the matching strand along with the leading strand. Later, another enzyme replaces the RNA primer with DNA.
The other original strand is the lagging strand. This is because this strand is opposite the direction the DNA polymerase extends the primer, so DNA synthesis occurs in short, unconnected segments. Enzymes fill in the strand and connect the short segments.
Replisomes move away from the replication origin along the DNA in both directions. The result is the replace the old DNA double helix with two identical ones.
Semiconservative replication occurs – one strand is old, the other is new. Once replication completes, two new chromosomes are in place of the original one.
DNA is surrounded by proteins. Sometimes the proteins wrap the DNA into the chromosome, which is only visible at mitosis. During interphase, DNA/proteins are loosely arranged in stringy masses.
Any new DNA strands must be exact complements of the parental strand.
Mutations are any change in a cell’s DNA sequence. They can be silent (nonharmful), harmful, or lethal. They are inherited by the daughter cells.
The first line of defense is DNA polymerase, which proofreads its own work. It checks to see if the base is the correct one; if not, the enzyme removes the nucleotide and replaces it with the correct one.
Cells also need to detect/repair mutations from during replication or caused by environmental factors (mutagenic chemicals/radiation). Most mutations are mismatches – the bases do not complement each other.
Cells fix mutations using excision repair. An enzyme recognizes the mismatch and binds to the DNA. Then it breaks the sugar-phosphate bonds of the mismatched section and removes the damaged/mutant DNA. Then DNA polymerase fills in the deleted DNA sequence. Then another enzyme forms sugar-phosphate bonds between the replacement piece and its neighboring nucleotides.
Sister chromatids (2 copies of each chromosome) are made during the S phase. They are attached at the centromere.
Chromosome segregation is the separation of sister chromatids; each new nucleus receives a copy of each chromosome. A mistake here results in one nucleus with two copies of a chromosome and the other nucleus with none. These abnormal numbered chromosomes in daughter cells are called aneuploid.
Mitosis consists of four steps.
In prophase, the nuclear membrane breaks down into small vesicles and chromosomes condense. Microtubules form around the nucleus and join to form a mitotic spindle. If he cell has centrioles (which are duplicated during interphase), microtubules form between them and push them to opposite poles of the cell (Plants don’t have centrioles). The microtubules at the ends of the spindle are anchored to protein structures surrounding the centrioles. (spindle poles). Within the centriole is a kinetochore (protein complex). Microtubles in the spindle bind to the kinetochores of each chromatid (Each chromatid is connected to a spindle pole). Sister chromatids join to opposite poles.
In metaphase, motor proteins pull the chromosomes into a ring between the two poles (metaphase plate), which is perpendicular to the spindle. This helps keep the sister chromatids attached at their centromeres.
In anaphase, enzymes break down the centromere. The motor proteins of their kinetochores pull them along the spindle microtubules to opposite spindle poles, making the chromatids into chromosomes.
In telophase, the chromosomes start expanding and the nuclear envelope forms around them, which produces two nuclei. Cytokinesis divides the cell as the plasmat membrane constricts between the nuclei.
In cells that have cell walls, vesicles containing cell-wall material fuse across the cell plate to complete the cell walls and cell membranes. Then the cycle repeats.
In most animal cells cytokinesis begins during anaphase. In plants’ cytokinesis, vesicles containing cellulose begin to congregate between the two nuclei, which begin to fuse and form the plasma membranes of the new daughter cells. Then the cellulose in the vesicles completes the two new cells. In fungi (yeast), the nuclear envelope forms a bud instead of breaking down. Spindle poles are embedded in the nuclear membrane.
Diagram Notes
- DNA has a negative charge. The nucleosomes were like the “beads” on DNA. The nucleosome is made of eight proteins (histones). They have positive charges that balance the DNA. DNA synthesized during the S phase binds to histones to form nucleosomes. The DNA wraps twice around the edge of each protein disk.
- Wherever the DNA contacts the histone, arginine (amino acid) occurs. The R group of this amino acid balances the DNA’s negative phosphate groups.
- DNA bound to nucleosomes is called chromatin.
- Long strings of nucleosomes form thicker coils to make up visible chromosomes through a light microscope. This excludes the enzymes involved in gene expression. Enzymes can modify the histone bound to specific genes, loosening the chromatin structure and activating DNA.
- In unicellular eukaryotic cell division, it’s similar to prokaryotes. Instead of attaching to the plasma membrane, though, the chromosomes attach to the nuclear envelope. The spindle forms in the cytoplasm.
- In yeast, the nuclear membrane never breaks down. It helps stabilize the spindle. In other unicellular eukaryotes, bundles of microtubules penetrate the nucleus.
- In more complex eukaryotes, the nuclear envelope breaks down during prophase and reforms after chromosome segregation.
- As microtubules became more active in separating the chromosomes, the nuclear envelope became less important in mitosis.
- The nucleosome is the basic packing unit of eukaryotic chromosomes.
Early cell-fusion experiments showed that something in S and M phases could cause G1 and G2 to advance to the next phase (like when S phase were fused with G1 cells, the DNA in the G1 nulceus began to replicate, whereas nothing happened with the G2 cells). M-phase cells can move G2 into mitosis.
When cells leave G0, cyclins begin to accumulate and rapidly disappear as the cycle progresses, regulating progression throughout the cell cycle. The most important of these are the G1 (reaching a peak during S phase then declining) and mitotic cyclins (reaching a peak at metaphase then declining). Cyclins act by binding to various kinases (enzymes transfer a phosphate group from ATP to other enzymes, which activates them). The kinsases’ quantity stays the same, but are active only when bound to the appropriate cyclin. The more cyclin, the more activation of kinsases. Mitotic cyclins stimulate kinases that activate the path that leads to breakdown of the nuclear membrane and condensation of the chromosomes. The last pathway activated is the one that reaks down specific proteins, which break the centromere.
Checkpoint control monitors the condition of the DNA, the chromosomes, and mitotic spindle of a cell during cell division. It’s made of proteins that detect mistakes and damage and stop the cell cycle until repairs are made (cell-cycle arrest).
P53 detects mismatched DNA base pairs and other DNA damage, preventing the G1 cyclin-kinase from bringing the cell into S phase. Yeast has a checkpoint that monitors the condition of the mitotic spindle; mitosis is then halted by inhibition of the mitotic cyclin-kinase system.
Regulators prevent cells from leaving the G0 stages. When they’re inactivated, cells may divide at the wrong time. Mutations in the genes that encode these proteins can lead to uncontrolled growth and reproduction, cancer.
Biology notes and summaries
Chapter 9 Biology Notes
- DNA and RNA make up genetic material.
- Gene expression is the reading and usage of genetic material.
- The storing of genetic information depends on two features of their molecular structure: repeating subunits that make up nucleic acids and the subunit bases of one strand pair with the bases of another strand.
- One strand acts as a pattern/template for a new molecule, which is built according to the plan stored in the original strand.
- DNA stores genetic information. DNA specifies the primary structure of proteins (sequence of amino acids). This primary structure determines a protein’s tertiary structure, which then determines the function of the protein.
- When a gene becomes active, an enzyme makes a temporary RNA copy of the information the DNA contains.
- mRNA is the temporary copy of a gene that encodes a protein. It is made in transcription. It provides the pattern that determines the order in which amino acids are added to a protein (translation).
- Protein synthesis takes place on ribosomes, which are made of proteins and rRNA. 80% of the RNA in a cell is rRNA.
- tRNA attaches to each amino acid that will be used in making the protein, one type of amino acid for each type of tRNA.
- There are also other nuclear RNA molecules that interact with proteins during RNA processing. Some RNA also act as catalysts. Viruses store genetic code as RNA.
- Genetic code describes how a sequence of bases in DNA/RNA translates into the sequence of amino acids in a protein. The nucleotides make up the DNA code. The genetic code needs at least 20 different code words: if they combined in groups of 1, only 4 amino acids would be coded; two would have 16 combos. However, if 3 are combined, there are 64 possible combinations. More than one genetic word can specify the same amino acid.
- Each nucleotide triplet in DNA directs a particular triplet to be formed in mRNA during transcription. A triplet in mRNA (codon) pairs with a triplet on a tRNA (anticodon). The genetic code is nearly universal.
- The start codon is AUG (methionine) and the stop codons are UAA, UGA, and UAG.
- Protein synthesis is the way that genetic information is expressed. They play key roles in the function of any living system. They make up cell structures/tissues. Keratin makes up skin, hair, and feathers. Collagen makes up connective tissues. Myosin helps muscles contract. Hemoglobin in red blood cells bind to oxygen.
- Proteins help in : structure, storage, hormones, antibodies, receptors, movement, enzymes, and transport.
- Hormones help maintain homeostasis; they are signals given off by cells in one part of an organism that regulate behavior of cells in another part. Insulin regulates blood sugar. Cell signaling involves protein receptors attached to the clel membrane. They pass along signals to a series of regulatory enzymes inside the cell (relay runners), which bind to specific DNA sequences/enzymes involved in transcription.
- Collagen exists as long fibers that bind cells together in tissues. Enzymes have cavities/pockets that bind only specific substrate molecules, like lysozyme, which destroys bacteria by cutting a polysaccharide found in bacterial cell walls.
- Genes are not expressed at all times. Only about 15000 out of 100000 genes are active at any time. This shows how cells play different roles in a multicellular organism. In the bacteria Escherichia coli help digest food in our body in response to a cellular signal that lactose is present.
- Gene expression begins with RNA synthesis. RNA polymerase joins RNA nucleotides according to the base sequence in DNA> Prokaryotes have one type of RNA polymerase; eukaryotes have 3.
- RNA is made in the nucleus, then modified and moved out to the cytoplasm through pores in the nuclear membrane.
- The nucleolus is where rRNA is made and also where rRNA and proteins assemble into ribosomal subunits.
- Only one strand of the DNA directs the synthesis of RNA.
- Transcription occurs in 3 stages: initiation, where RNA polymerase attaches to a specific region of the DNA (promoter region that promotes transcription). Proteins (initiation factors) are needed for it to attach. RNA is then elongated – RNA polymerase partially unwinds the DNA. A single complementary strand of RNA (primary transcript) is made. Then termination is when it reaches the terminator region (end of the DNA to be transcribed).
- The life span of eukaryotic mRNA depends partly on how the primary transcript is processed. A transcript can contain as many as 200,000 nucleotides, but since they are processed, the mRNA can average only 1000 in the cytoplasm.
- How mRNA is processed: Enzymes attach an mG cap (methyl-guanine) to the start of the mRNA. Then the end is replaced by a poly-A tail. They both help protect mRNA from enzymes that break down nucleic acids. The longer the tail, the longer the life span. It may also help transport mRNA out of the nucleus. The cap helps the mRNA attach to a ribosome and begin translation. The final step involves removal of introns (don’t code for protein), leaving the exons. The process of removing introns and rejoining cut ends is splicing, which requires precise recognition of the site to be cut (GU at one end and AG at the other).
- Protein enzymes catalyze the splicing of tRNA in yeast and other eukaryotes. Catalytic RNAs splice RNAs produced by mitochondria, chloroplasts, and unicellular eukaryotes.
- tRNA also needs chemical modification of several nucleotides and folding into a cloverleaf shape as well as splicing.
- rRNA is not involved in coding. The primary rRNA transcript is spliced/modified to produce mature rRNA molecules, which bind to proteins to form subunits of ribosomes.
- Protein synthesis occurs on ribosomes, where tRNA acts as a molecular adapter; the codon sequence of mRNA is translated into the amino-acid sequence of a protein. The anticodon pairs with the mRNA codon that encodes the particular amino acid.
- tRNA charging is attachment of the correct amino acid to its specific tRNA molecule (20 amino acids, 20 tRNA molecules). ATP provides energy. Charged tRNA, mRNA, and the polypeptide chain come together at specific binding sites on a ribosome, where tRNA anticodons base-pair with mRNA codons. This positions the amino acids they carry (bond to the chain).
- The P site holds the tRNA carrying the growing polypeptide chain.
- The A site holds the tRNA carrying the next amino acid to be added.
- An uncharged tRNA leaves the E site after its amino acid is added.
- The ribosome moves along the mRNA strand one codon at a time.
- Translation also occurs in the same stages as transcription. Initiation/elongation need energy from GTP.
- In initiation, a large and a small ribosomal subunit attach at the start codon.
- At elongation, peptide bonds join each amino acid with the next. A charged tRNA enters the A site of the ribosome. It base-pairs with the mRNA, position its amino acid to form a peptide bond. The bond forms, and the peptide chain bonds with the amino acids on the tRNA in the A site. The ribosome moves one codon along the mRNA, and the tRNA in the P site leaves the E site, while the tRNA in the A site goes to the E site.
- In termination, a stop codon reaches the A site of the ribosome. Instead of tRNA, a release factor binds to the stop codon in the A site, and then translation stops there and the tRNA releases the polypeptide.
- The protein chain is chemically modified (adding sugars or cut the polypeptide) and folded into tertiary structure. Helper/chaperone proteins stabilize the polypeptide when it’s folded.
- Pepsin and trypsin become active when they’re cut.
- When proteins need to move out of the cell, vesicles containing the protein fuses with the cell membrane, then the protein’s released outside. If the protein needs to become a membrane, signal sequence starts when it is being translated. These proteins are made on the rough ER. Their first few amino acids are the signal sequence, which binds to a receptor protein in the ER. During translation, the polypeptide goes through the membrane and into the ER. When translation completes, the protein is released from the ribosome into the inner ER (with a sugar molecule) to the Golgi apparatus (vesicles).
- Most commonly, translation errors come from misreading the nucleotide sequence. Initiation determines where translation will begin – the groups of codons are the reading frames. If the frame is shifted even a little, different codons/amino acids will result. Some erros are from splicing mistakes or changes in the DNA.
- Sometimes, translational frame shifts / alternate initiation sites are normal ways where mRNA can specify more than one polypeptide.
- Viruses are tiny particles that have no cells but replicate/evolve. They do this by using the gene-expression machinery of the host cells they infect. They contain a small amount of genetic material. The T2 bacteriophage stores it as DNA. The influenza stores it as RNA. This is protected by a protein coat.
- Some viruses hae a membrane envelope, but don’t carry out metabolism or respond to stimuli like cells.
- In lytic infections, the host cell’s enzymes replicate the viral DNA, and ribosomes translate/transcribe the viral DNA for its protein coat. When there are new viruses, the cell lyses (breaks open) and releases them to infect other cells.
- In lysogenic infections, the viral DNA inserts into the cell DNA and copied; there is no production of viruses, but the viral DNA is copied and passed down.
- Starvation of the host cell can then activate a lytic cycle of replication. When the viral particle comes, it can pick up part of the cell membrane. In tumors, the cells can lose control of growth.
- Antibiotics are useless against viruses because viruses do not have metabolism.
- Reverse transcriptase makes a DNA copy of the viral DNA when viruses infect a host cell. Then the viral DNA joins the cell’s DNA and directs production of new virus particles (ex: HIV). Retroviral genes generate new copies of DNA from RNA and insert them at random into the host chromosomes.
Biology notes and summaries
Biology Chapter 13 Notes
- Siamese cats’ fur has enzymes that produce a dark pigment, which function best at lower temperatures. This shows that the environment has a strong impact on gene expression.
- Identical twins develop when a zygote forms two complete embryos, and have the same genes. Fraternal twins come from separate eggs and sperm cells, and are not identical. If identical twins exhibit the same trait more than fraternals, then genetic factors influence it. But if traits differ in identicals, then the environment influenced it.
- “Blending” inheritance – An individual’s genetic makeup was formed when the parents’ genes mixed at fertilization, which resulted in averaging the parents’ genes. When the parents’ contributions were blended, they could not be passed on separately to future generations. (No longer included in genetics, however, it was a logical explanation or visible differences. However, it couldn’t explain why kids looked like grandparents or other relatives more.
- Mendel used garden peas to study heredity. He chose peas because they’re easy to grow, and could grow a lot of generations during 8 years; they also self-fertilize. Self-fertilization is done in peas when: the petals of the flower completely enclose the reproductive organs, so pollen from the anthers falls on the stigma of the same flower. Pollen tubes grow down through the female reproductive organ to the ovules (ovary), which develop into seeds; the ovary wall develops into the pea pod.
- Mendel didn’t study traits that show continuous variation (seed size), but concentrated on traits that didn’t fit “blending”: color, general height, surface texture, etc.
- True-breeding plants always produce identical offspring (to themselves). Mendel ensured he had true-breeds. Then he crossbred his plants and classified the offspring, looking for patterns of inheritance. The blending theory didn’t explain his results (yellow + green = … all green? O_O).
- Mendel called these passed down traits genetic “factors” that remain separate generation after generation. Some factors remained hidden in some generations, but were still present and able to act. However, they were not “blended” into the green pod factor)
- The concepts of genes now has replaced Mendel’s factors. A gene is a segment of DNA whose sequence of nucleotides codes for RNA and proteins (functional!)
- Most genes exist in more than one form (allele); each allele of a gene has a different base sequence (like Mendel’s factors).
- All organisms have genes that exist as several different alleles.
- Widow’s peak is from the gene affecting the shape of the hairline (tapering to a point above her nose). Others have smooth hairlines. Male pattern baldness is a gradual loss of hair on the top of the head.
- Most traits scientists once thought were determined by single genes result from the interaction of several genes with each other and the environment.
- Eukaryotic chromosomes are long molecules of DNA wrapped around proteins, and only part of this DNA codes for proteins; the noncoding part is not translated. Some noncoding DNA consists of short repeated base sequences. 1% of eukaryotic DNA is expressed as protein.
- In prokaryotes, DNA is a single circular chromosome with little associated protein. Plasmids, small circles of DNA containing additional genes, are also there. They can move between bacteria. They can also carry genes resistant to antibiotics. They don’t usually have introns; 90% of DNA is translated.
- Homologous chromosomes carry the same genes, though the genes can be present as different alleles. Homologous chromosomes can be most distinguished (aside from size and position of centromere) by staining it. The stains bind to specific regions of chromosomes, creating banding patterns that are specific to each of an organisms chromosomes. Chromosome painting is another technique that can be used to identify quickly all of an organism’s chromosomes.
- Chromosomes are easiest to study when they’re condensed. White blood cells are used most frequently to study human chromosomes – they can divide and grow easily. Chemicals are used to stop cell division during metaphase. Then they’re treated with water, which makes them swell and makes the chromosomes spread apart. Then they stain the chromosome spread, resulting in banding patterns.
- Karyotypes are photographs of stained chromosomes which are cut out and arranged by size and shape. Karyotypes of fetal cells can be used to check for suspected chromosomal abnormalities.
- Probability is math that predicts the chances that a certain event will occur. It’s usually expressed as a fraction. The chance that 2+ independent events will occur together is the product of their chances of occurring separately. Math tests let geneticists see if the observed/predicted results is because of chance or something else. The larger the sample size, the less deviation you would expect from predicted ratios.
- A monohybrid cross is a difference in one trait color. The first cross plants are the parental (P) generation. Their children are the first filial (F1) generation. The children’s children are the second filial (F2) generation. According to Mendel, the dominant trait was the one trait visible in the F1 generation, and the alternative trait that was not visible was the recessive trait.
- Mendel said that each true-breeding plant has two identical copies of the factor for a trait, and when gametes formed during meiosis, only one copy of the factor went into each pollen or egg cell. At fertilization, the F1s received a green-pod factor and a yellow-pod factor, and only one of those went into each gamete formed by the F1 plants – the principle of segregation. In short, the alleles of each gene separate during meiosis when homologous chromosomes are divided among the gametes.
- Dominant alleles are represented by a capital letter, and the recessive by lowercase. Pea plants are diploid (like most multicellular organisms), so their genotype (genetic makeup) would consist of two letters.
- At fertilization, the babies receive one gamete each from their parents, so it can be a mixup: GG + gg -> Gg
- Examples: GG, gg = homozygous, Gg = heterozygous. The genotype determines the phenotype (visible appearance/observations)
- A Gg + Gg -> 1 GG : 2 Gg : 1 gg
- A Punnett square helps calculate probable ratios of genotypes/phenotypes. The genotypes of one parent are on one side; the genotypes of the other are on top.
- A dihybrid cross shows crosses between individuals that differ in two traits. The two genes in this cross are on different pairs of homologous chromosomes. Their inheritance is not connected. Whether a particular gamete receives a R or r allele has no effect on whether it will receive a chromosome that carries a Y or y. They are inherited independently. This is Mendel’s principle of independent assortment: Alleles for one characteristic divide up among the gametes during meiosis independently of alleles for other characteristics.
- In humans, mammals, and the fruit fly, sex chromosomes are X and Y. Females – XX, Males – XY. All the eggs produced in meiosis have X chromosome, and only HALF of the sperm produce Y (while the other half produce X). Sperm determines the offspring’s offspring. If a sperm that has an X chromosome fertilizes the egg, then the result is a female, and vice versa for the Y.
- Grasshoppers and some other insects have XX females and XO males. Birds, fish, and some insects have Z-W system. Male = ZZ, female = ZW. Some plants have separate female and male plants and have sex chromosomes that are like the X-Y system. Most plants and some animals have no sex chromosomes.
- Some genes show incomplete dominance, like white and red snapdragons crossing to make pink ones. Codominant alleles are two dominant alleles, like AB in AB blood. Multiple alleles are the existence of several alleles in a gene. For example, A blood can be AA or Ai. The i, when combined with another person’s Bi or ii, can produce O blood.
- O is the universal blood donor, and AB is the universal receiver. However, you can’t donate AB unless it’s to another AB, and O can only receive O. You can’t donate A blood to B blood because blood w/ill clump because of antibodies, defensive proteins in the blood that go against the specific carbs found in the other blood.
- Linked genes are on he same chromosome and are inherited together. However, the alleles don’t always stay together because of crossing over, which increases genetic variation. The father apart two genes are on a chromosome, the more likely a break will occur between them, and if they’re far enough apart, the breaks might be so frequent that they assort independently. Frequency of linked traits separated ~ distance from each other on the chromosome. This can be used to map the positions of genes on chromosomes. Important genes that are hard to identify can also be mapped by observing traits produced by nearby marker genes.
- Huntington’s disease is a congenital nervous system disorder.
- X-linked traits were first shown by Bridges and Morgan and Sturtevant. They only found white-eyed males for a long time. When they crossed the white eyed females with red eye males, the F1 was only red-eyed females and white-eyed males.
- Morgan said that the white-eyed traits were only carried in the X chromosome; since males only have 1 X chromosome, whatever genes they get that are associated with X are expressed. Since females have two X chromosomes, they can express a mixture of X-linked traits. X-linked traits are usually recessive.
- Examples of X-linked traits are red-green color blindness and hemophilia (irregular blood clotting).
- Lyon said that early in the development of a normal female, one X chromosome becomes inactivated in each somatic cell. It’s random! In some cells, the X-linked genes from the mom are expressed, but in others, the X-linked genes from the dad are active. This explained a Barr body, a dark mass in the nucleus of female cells. It’s the tightly condensed, inactive X chromosome.
- Nondisjunction is the failure of homologous chromosomes to separate in meiosis, resulting in an extra or missing sex chromosome.
- Human disorders resulting from nondisjunction are: Turner syndrome (XO – short, sexually underdeveloped, infertile females), Klinefelter (XXY – tall, sexually underdeveloped, slightly mental males)
- Trisomy is three copies of a chromosome; trisomies of autosomes (chromosomes other than sex chromosome) disrupt development and the embyo dies. Trisomies of human chromosome 21 causes Down syndrome 9 mental, short, characteristic facial, heart defects, with varying severity. 95% reach adulthood, 80% of those live into early 50s.
- Multifactorial traits are affected by genetic and environmental factors. They include height (quantitative traits) and the environment (light, temperature, metabolic activities). Melanin is thought to be controlled by four major genes that are codominant. Independent assortment of the alleles of these genes results in continuous variation in skin color, and exposure to sunlight also affects it.
Biology notes and summaries
Biology Chapter 19 Notes
- Descent with modification is long-term change in inherited characteristics (evolution).
- Different species arose as a result of inherited variation in populations, giving some an improved chance to survive/reproduce.
- Evidence of evolution includes fossils, the record of ancient organisms. The fossil record in different strata of the earth shows the order of evolutionary change. Palentology is the study of fossils.
- Examples of the appearance of new species from ancestral ones include diatoms, singled-celled photosynthetic organisms that live in water, have hard shells that can be preserved as fossils.
- Harder parts of animals, like bone, survive decay. Harder minerals replace the original substance of animal parts and preserve them as fossils. Softer tissue leaves an impression on soft mud that dries. Ancient insects were preserved when trapped in tree sap that became amber.
- The fossil record, though it contains about 300,000 species of fossils, is incomplete because most dead organisms decay and do not become fossils.
- Fossils offer physical record of organisms not found on earth today; you can compare fossils in younger, shallower rock with that of older, deeper rocks.
- Extinction occurs much more often because of increasing human population, which in turn changes the environment, such as the passenger pigeon. Also, overhunting can be an issue.
- Fossils record organisms before/after new species split off from older ones. They show the rate of evolutionary change and show intermediate stages between species and their ancestors. They have uncovered “feathered dinosaurs”, suggesting the birds evolved from dinosaurs.
- While Darwin was aboard the beagle, he examined many things, including the Galapagos tortoises. On islands where cacti, its food, grew high on tough woody stems, the tortoises’ necks were long and their shells were saddleback. On islands where the cacti is low-growing, the tortoises are normal necked and shelled. This shows coevolution, the continuous adaptation of different species to each other.
- This is also found in the adaptations of predator and their prey: for example, we have bitter tastes and don’t like it: this protects us from plant toxins. Symbiotic species have coevolved to the point where neither can survive without the other.
- Artificial selection is one way to drive evolution experimentally: breeders choose who can mate, however. Natural selection states that members of a population members with the most adaptations to their environment are most likely to survive and reproduce.
- Moth-eating birds was responsible for changes in the colors of peppered moths.
- Dice conducted an experiment with buff-colored or grey mice and barn owls. Dice alternated the color of soil on the floor of a room each day with sticks. 4 mice of each color were released and exposed to an owl for 15 minutes. One set of mice, depending on the soil color, survived up to twice as much as the other set of mice.
- Homologies are similarities in the structure of an organism, like anatomy, behavior, and its polymers.
- Homologous genes are responsible for the formation of the body plan and organs even in very distantly related species.
- Since inheritance was poorly understood, he couldn’t defend his theory of evolution by natural selection.
- Variation is found in mutations and the recombination of alleles in sexually reproducing eukaryotes (crossing over, independent assortment, and fertilization).
- Genetic variation is the raw material of evolution.
- Gene duplication produces gene families (multiple copies of nearly identical DNA sequences). Some of the copies, pseudogenes, are not expressed; they are not subjected to natural selection. This means that pseudogenes accumulate mutations faster than functional genes in the same family.
- Scientists compare amino-acid sequences and nucleotide sequences of homologous proteins and genes. An example is the a and B form of globin. They are very closely similar. Anatomy and these genes support the hypothesis that all vertebrates share a common ancestor. This is also shown in Hox genes.
- Many strains of bacteria that were killed by antibiotics have evolved to be resistant, such as tuberculosis. The more they’re exposed, the more the new population will be resistant. This has also led to pesticide-resistant insects.
- Mutation is common in short repeated nucleotide sequences (microsatellites). They increase genetic variation. This occurs in the bacteria Neisseria gonorrhoeae and determine whether the bacteria can infect a person and case gonorrhea. Some cause the disease and others survive without causing it.
- The microsatellites ensure that at least some bacteria can survive.
- Speciation is the appearance of a new species. They include hybrids, such as the breeding of a Labrador and a German shepherd to create a mutt. Artificial selection includes them, such as creating new fruit flies by exposing some species to radiation.
- Populations evolve, not individual organisms within a population. Genetic changes indicate that evolution is taking place. A gene pool that is in a state of equilibrium has a frequency of alleles that tends to remain stable.
- Special in sexually reproducing organisms occurs when two populations become so different in their genetic makeup that they can no longer interbreed. Sometimes new species out-compete and replace the species that they originated from. Mostly, a small population is isolated from the rest of species and develops into a new species.
- Geographic isolation (water, mountain range, impassable canyon) (Ex: tufted-ear squirrels)
- Ecological isolation: two populations adapt to different habitats (willow flycatcher – brushy slopes; alder flycatcher – alder swamps)
- Mating behavior and physical characteristics are important in the reproductive success of some organisms: sometimes gametes are not chemically compatible. Also, differences in size of the organisms or size/shape of their reproductive organs.
- Behavioral isolation – mating pattern of a small group of organisms becomes different from that of the main group (reproductively isolated) (Ex: leopard frog – differences in premating behavior)
- Seasonal isolation - The leopard frog populations mate at slightly different times during the year. This occurs in plants and animals – flowering at different times (pollen cannot pollinate carpels)
- Isolation mechanisms – those that occur prior to and after zygote formation (Prezygotic – geographic isolation, mismatched mating behavior, seasonal isolation. Gametes never meet/fuse. Postzygotic – failure of the zygote to develop normally due to differences in parents).
- Matings between individuals of related species often produce offspring that cannot produce normal gametes, making for an infertile thing.
- Polyploidy is duplication of chromosomes that often makes a new species of plant. Offspring cannot mate successfully since other plants have the parental genes. This forces them to reproduce asexually or forces them to mate with other polyploidy offspring, establishing a new species.
- Cross-species hybrid plants reproduce asexually. In time, a chromosome duplication in a hybrid plant can produce a tetraploid new species.
- When a population enters an environment with few competing species, it often divides into several smaller populations, which avoid competing with each other by adapting or by using different resources in the same habitat. Then each population can become a new species. (Adaptive Radiation)
- Stasis is the large-scale change that remains very slow for a long period of time. Sometimes “living fossils,” which have changed very little from their ancestors, appear, like lungfish and the horseshoe crab. These live in an isolated, stable environment with little competition.
- Gradualism is when evolutionary change/speciation occurred through gradual and fairly constant changes.
- Punctuated equilibrium involves a short period of rapid change just after a population becomes isolated and forms a new species, and then slows down and approaches stasis.
- These two patterns are extremes in evolution.
- Selective pressure acts on the raw material of genetic variation to produce heritable, long-term change in living populations.
- Hurton (chemical/physical processes as one rock changes into another), Smith (cut canals and observed strata with embedded fossils), and Lyell saw geologoical time. More evidence is found in radioactive decay. The Rb-87 isotope decays very slowly into Sr-87. The ratio of these isotopes in a rock/fossil is used to calculate its age. (and with others as well)
- An “icebox fossil” was recovered whole from Siberia in 1999. It was a soft-tissue woolly mammoth. It was preserved very well. It is called the Jarkov mammoth.
- Japanese hope to revive the mammoth. They will try to crossbreed a mammoth with an elephant by trying to remove the sperm intact. The mammoth, however has 58 chromosomes while the elephant has 56, even though they share a close genetic homology. DNA are about 5% different.
- They could also try to use DNA or a cell nucleus to try to clone the mammoth.
- The fossil record provides evidence of the rate of evolutionary change. They indicate that anatomical structures do not change at a constant rate. It’s more constant for a particular DNA or protein sequence. But the rate for a functional part of a protein is slower than a change in the less functional parts of the molecule (like the surface parts).
- A unit of evolutionary change is called a darwin, which is the change in a characteristic being studied by a factor of e (2.718) in 1 million years. They can measure it since it’s possible to count the number of changed bases in DNA or amino acids in a protein
- A species can become extinct if its habitat has been destroyed or its environment changed. Evolutionary changes can also affect other species in the same environment. Sometimes, environments have changed so quickly that a lot of species died out.
- The last mass extinction occurred about 65 million years ago: the climate cooled, and a lot of things died out. At least two asteroids or comets struck Earth while these extinctions occurred. (Iridium). Alvarez studied it and concluded it was from dust. However, the extinctions, though large, were not abrupt.
- Advantages to mass extinctions: vacated habitats, ensures survival/dispersal for the survivors
Biology notes and summaries
Chapter 24 and 25 Notes
- Ecosystems make up the biosphere. Limiting factors (abiotic) limit species in many ways.
- For example, temperature limits bacteria and fish that have adapted to high and low temperatures, respectively.
- The availability of water makes some organisms, like fish, have some adaptations. Ocean fish actively transport salt from their body, while freshwater fish excrete excess water. Land organisms adapt to conserve water and salts. Desert organisms are most susceptible. Cacti have thick cuticles. Small desert mammals are active at night.
- Sunlight limits water organisms. Most photosynthesis in water, therefore, occurs near the surface of the water.
- Physical structure, pH, and mineral composition of soil limit the distribution of plant species and their eaters. Forest soils contain small amounts of nitrogen and phosphate that plants need for amino acids / etc.
- Two adaptations for forest plants to survive on these soils include fungi symbiosis (absorb phosphate), and networks of shallow roots that absorb nutrients from decaying organic matter in surface soils (before they wash in deep).
- Wind carries off heat energy and speeds up evaporation. They affect how plants grow.
- Catastrophes can remove organisms temporarily from an area, which is repopulated by survivors or outside organisms. Like in CA, shrubs survive by storing food in fire-resistant roots.
- Plant roots, lichens, and microorganisms help break down rocks into soil. Dead material and animal wastes add organic matter and nutrients to soil. Roots/organic matter hold soil together. Transpiration increases humidity (rainfall/clouds).
- Adaptation to one environment generally means exclusion from another. Ex: Desert saltbush likes dry soil, Oaks like light, Lake trout likes freshwater, etc.
- Saltbush can tolerate high salt levels because it accumulates salt in epidermal bladders on its surface. A small stalk puts the salt in the bladders from leaves. Eventually the bladders burst. It concentrates the Na and Cl it absorbs. Beets and tomatoes are also pretty salt-tolerant.
- The trophic structure is the nutritional relationships among the producers/consumers in an ecosystem. Producers support the system, while the other levels include consumers that depend directly/indirectly on the producers for energy and nutrients.
- Herbivores are primary, like deer, grasshoppers, and snails. Carnivores are secondary. Then tertiary consumers may eat them, like the hawk eating the bird that eats the insect that eats the plant. Decomposers consume organic wastes and dead organisms from all levels. The 10% rules states that only 10% of the energy at each trophic level is available at the next highest level. Plants have the most energy.
- Land producers: plants; aquatic: algae (photosynthetic protists/bacteria)
- Mostly bacteria and fungi are the primary decomposers. They secrete acids/enzymes that digest complex organic compounds (cellulose) and then absorb the smaller digested products. Earthworms and scavengers are also decomposers.
- An energy pyramid shows the quantity of energy each type of organism can receive at what level of the trophic structure.
- Biomass is the total quantity of living matter at each trophic level. It also declines at higher trophic levels. For example, wolves are less than rabbits. Productivity is the rate at which new biomass forms. It also declines at higher trophic levels. An ecosystem can sustain few top-level carnivores.
- Meat and other animal products are concentrated sources of protein/energy, but provide only a small part of the energy fixed by photosynthesis.
- Predation is when a predator consumes a prey and moves energy/nutrients through food webs. When two species consume the same thing, they compete; this is important in natural selection.
- A niche is a particular combination of resources that a species is adapted to exploits. For example, some animals live in treetops and eat fruits, like birds or monkeys. Since they share the same things, they compete.
- The competitive exclusion principle says that two species cannot occupy the same niche in the same ecosystem for long. The species that competes more successfully for food, space, or other resources displaces its competitors. Adaptive radiation reduces competition. Adaptations let competing species divide or share resources and reduce competition. (Ex: hawks hunt by day and owls by night even though they eat the same stuff).
- Symbiosis is when two species live together. Mutualism is when both benefit, like the legume (carbs) and bacteria from Rhizobium (N compounds). Parasitism is when a parasite gets nutrients from a host, like viruses and mistletoe (draws water/dissolved soil mineral nutrients from xylem of tree). Commensalism is when one organism benefits and the other is unaffected, like algae and barnacles.
- Close contact between symbiotic organisms can lead to an occasional exchange of DNA, like chloroplasts/mitochondria and parasites that get their host’s genes.
- Oragnisms absorb and release carbon/oxygen/nitrogen as gases. They move through cycles. Phosphorus and sulfur are not global.
- Carbon cycle: carbon fixation/respiration move carbon through the biosphere. Autotrophs absorb CO2 and reduce it to form compounds. Some of the carbon remains in bodies. Respiration by everything returns the carbon to the air. Fossil fuels also release CO2 into the air.
- Only some prokaryotes convert atmospheric nitrogen to ammonia (nitrogen fixation). Then more bacteria convert it to nitrite or nitrate (nitrification). Plants absorb ammonia or nitrate from soil/water and use it to synthesize nitrogen-containing compounds. Consumers and decomposers obtain their nitrogen from other organisms. Denitrifying bacteria convert nitrate to N gas.
- The water cycle shows that plants absorb water from the soil. Land animals/consumers/decomposers absorb water from their food or drink it. Aquatic animals are always in water. Water returns to the atmosphere through transpiration, cell respiration, and evaporation (mostly from oceans). Water vapor condenses and returns to the system as precipitation.
- Various factors limit the productivity and populations of ecosystems. Grasslands: nitrogen, Arid: water/nitrogen, Water: desert, Predator: Prey
- Populations rarely grow exponentially; when it grows, its population density (# / unit of land area or water volume). Competition increases; resources decrease. Toxic wastes may accumulate, and predators/parasites may rise up. This reduces reproduction and increase death rate, slowing population growth. Logistic growth is when it has an exponential, linear, and then stable maximum phase.
- The maximum is carrying capacity, the largest population that the environment can support. They are not fixed and change with factors like food supply.
- In species that reproduce rapidly, exponential growth can lead to a brief period when the population exceeds its carrying capacity, then they reproduce much less and die much more. (boom-and-bust cycle)
- Predator-prey cycle says that the cycles of the predator populations rise and fall with their prey.
- Biomes are groups of similar ecosystems, usually defined by the most conspicuous types of vegetation. The main climatic factors affecting the geographic distribution of biomes are the climate, temperature, and precipitation at different altitudes and latitudes.
- Increasing altitude is similar to increasing latitude in terms of temperature. The same biomes can be in very different places. A landscape is differences in soil, topography, and local disturbances resulting in a patchwork of interacting ecosystems.
- The tropical rainforest occurs on lowlands near the equator with intense sunlight and rainfall and evaporation (high humidity). C. America, Amazon, Ivory Coast, C. Africa, Madagascar, S. India, Ceylon, Thailand, Indonesia. It has little light on the ground because of the canopy, limiting the growth of vegetation. They are the most complex of all communities and support the most species. They are poor in nutrients like N and P. Deforestation has removed more than half of the rainforests for grazing land or farmland.
- The savanna is a tropical/subtropical grassland with scattered trees and woodlands. They are in areas with low/highly seasonal precipitation. They are usually in the interior of continents. Brazil (high plateau), c. Africa, w. Madagascar, n. Australia. It has 3 seasons : cool and dry, hot and dry, warm and wet. They are richer than the rain forest. They are home to some of the world’s largest herbivores, like elephants, giraffes, etc. However, a lot of poachers have killed them.
- Deserts have severely hot/dry climates. N. Africa (Sahara), They have almost no perennial vegetation. In less arid areas, predominant producers are scattered shrubs and cacti or other succulents (thick leaves with large cells able to store water). There are short heavy rains. Seed-eating herbivores like ants, sparrows, and rodents, are common. Lizard and snakes are important predators.
- Chaparral are brushland communities in midlatitdue coastal areas near cool ocean currents (mild/rainy winters and long/hot/dry summers). They have dense, spiny shrubs with tough leaves, often coated with a thick cuticle (increases flammability). CA, Chile, SW Africa, SW Australia, Mediterranean. They are adapted to and maintained by periodic fires. They store carbs and minerals in roots which regenerate. Species produce seeds that germinate only after a fire. Deer, fruit-eating birds, rodents, lizards, snakes.
- Temperate grasslands are similar to savannas, but prairie trees are found only near streams. They expanded in areas when climates became warmer/drier after the last ice age. Grazing animals like bison upped population. Speed and endurance are found in animals. Three grasslands in NA: tall-grass prairie (Midwest), short-grass prairie (E. of Rockies à Kansas), mixed-grass (Great Plains). Rich soils and favorable climate = agricultural. Cattle ranching/raising crops.
- Temperate deciduous forests are in midlatitude regions (enough moisture for large trees). Rain and snowfall. High humidity. High growth/decomposition rates. Fertile soil. 5-6 month growing season. Several layers of producers (short herbs/intermediate shrubs/tall trees). E. NA, w. & c. Europe, e. Asia, e. Australia à Tasmania. They support diverse animal species. Birds and small mammals. Larger predators (wolves/mountain lions). Soil/climate for agriculture, so lots of clearing in N. Hemisphere.
- Taiga is northern coniferous forest. N. NA, Europe, Asia north to the tree line. High elevations. Harsh winters; short, cool summers. Snow is main precipitation. Dominated by conifers that absorb most of the sunlight. Mosses, lichens, shrubs.
- Tundra is beyond the tree line or very high altitudes. Plants are compact, shrubby, matlike. In the Arctic tundra, permafrost prevents roots from penetrating into the soil. It blocks drainage (always soggy). Only shallow roots of small plants. Spring is in mid-may; only plants remaining near the sun-warmed ground can grow/flower/produce seeds. They receive less precipitation than most deserts. Daylight lasts almost always. Shrubs, sedges, grasses, mosses, lichens.
- Alpine tundra occurs above the tree line on high mountains. Many of the same species are in Arctic/alpine tundra. Brief/intense periods of productivity/plant growth/reproductive development in summer. Long winters / heavy snowfall / winds.
- Most of the earth’s biosphere is aquatic. There are two: freshwater (rivers/ponds) and marine (oceans/estuaries) ecosystems. At the source of a river, the water is cold, clear, rapid, low salt, few nutrients. Downstream, when it joins an oean/lake, the water becomes more turbid and slow. Upstream, the communities are simple and live in water with high levels of dissolved oxygen and low temperatures. Downstream, the oxygen levels decrease and temperature increases and it’s more complex.
- When a river/stream enters a lake, the speed of the current decreases and suspended particles settle. The amount of material remained suspended and the amount of light scattered by the particles determine photosynthesis depth.
- Phytoplankton (cyanobacteria and photosynthetic bacteria) near the surface: P and N limit them. Zooplankton (protists/small animals) feed on phytoplankton. Small fish, aquatic insects, and mollusks feed on plankton.
- The distribution of marine organisms depends on the penetration of light (depth/clarity). Photic zone – shallow top layer (photosynthesis). Aphotic zone (insufficient for photosynthesis: obtain energy by consuming living/death organic material produced in photic zone or chemoautotrophic bacteria)
- Intertidal zone – shore between high-tide and low-tide marks. Neritic zone – shallow water over the continental shelf. Pelagic zone – open water not associated with the seafloor. Benthic zone – seafloor. Abyssal zone – area of seafloor where light doesn’t penetrate.
- Organisms can disperse. Planktonic larvae and seeds are carried by wind or animals. Competition makes this happen. Sometimes the dispersing organisms encounter and unoccupied habitat and settle in, or colonize, the new area.
- Humans disperse exotic plants and animals. Often they perish, but sometimes it takes well to the area and establishes itself. Examples are kudzu, zebra mussel, fire ants, and African bees.
- If conditions of the physical environment change or if new species move into established communities, then the entire ecosystem can change dramatically. Ecosystems change when the environment changes.
- A community goes and a new community moves in (succession). Lichens grow first, then mosses, then ferns and seedlings, and finally into spruce and fir. Primary succession begins on bare rock, on glacial deposits, or in a lake bed. Secondary succession is in a disturbed site where soil is already present. Early stages are dominated by plants that disperse easily, grow rapidly, and have adaptations for colonization. Annuals start them. Colonizers tolerate a wide range of stresses and then are replaced by perennials. The types and number of species change. It depends on which species colonize an area in the early stages and on environmental disturbances.
- The process of succession is driven mostly by the organisms. In Mt. St. Helens, an orderly succession and a chance succession occurred.
- The more succession, the more interaction (predation/competition), opening up new niches and increasing the diversity of species. A climax community is when the web of interaction becomes so intricate that no more species can fit into the community unless others disappear.
- Most landscapes are a patchwork of communities in different stages of succession because of disturbances; biomes are climax communities reflecting the climate, soils, and topography of an area. Where disturbances recently occurred, good colonizer species are common.
- Species diversity may be greatest when disturbances are fairly frequent but not too severe. The north temperate-zone forest have shifted from taiga to deciduous because of warmer temperatures.
- Ecosystems provide natural resources that are essential for life. People change habitats in ecosystems to increase the yield of certain resources. The production and processing of natural resources employ more people than any other economic activity on Earth.
- Ecosystems modify climate (transpiration/absorbance of solar radiation: vegetated ecosystems = moderate temperature/stable climate). They prevent erosion and build soil (roots/leaf litter). Ecosystems break down wastes (domestic/agriculture wastes released into ecosystems that decompose organic wastes and purify the water and air). Ecosystems store carbon and maintain the carbon cycle. Energy flow maintains the cycle and stored over 500 billion metric tons of carbon in forest organisms and soil’s organic matter.
- Common pool resources are goods/services provided by ecosystems that are shared by many but controlled by no one. They are exploited by competing parties, all of whom profit. They are, however, controlled, so that people don’t get too greedy.
- The Value of the World’s Ecosystem Services and Natural Capital stated that the goods/services are infinitely valuable to human services.
- Humans have become a major force in changing the natural landscape. Less-developed countries have more people than more developed countries. We just passed the 6 billion mark. 8 billion is expected by 2050.
- Our impacts are local and global. The most important local ones involve land use. Agriculture is very disruptive. These are managed ecosystems that do not contain the typicall/naturally found organisms of the region. The natural habitat is destroyed. Biodiversity is decreasing. The effects are global.
- Local air pollution harms hujman health. E. Europe lacks air-pollution controls. Acid rain affects larger regions (fossil fuels) and add large amounts of S and N oxides, which react with water and turn into acid rain, which destroys life in lakes.
- CFC (Chlorofluorocarbon) use has led to a serious decline in the ozone layer of the stratosphere. Human activities have added a number of gases to the atmosphere, which contribute to the greenhouse effect, which traps infrared radiation and cause the atmospheric temperature to rise. CO2 has increased a lot.
- The greenhouse effect is responsible for Earth’s warmth. Temperature will be higher, rainfall will change. Sea levels will rise. Organisms might not adapt fast enough.
- People should cut back on oil, coal, and gas, use energy more efficiently, and make more use of renewable energy sources.
- The ozone layer absorbs UV rays. It originates from interaction of UV rays with atmospheric oxygen. A free radical is in Chlorine, which is in CFCs. The chlorine radical can act as a catalyst that breaks down more ozone.
- Scientists reported an ozone hole over Antarctica, which were less than half their usual concentration. In summer, it breaks up. The Montreal protocol phased out CFCs.
Sources :
http://www.freewebs.com/altara2s/Biology%20Chapter%201%20Notes%20%282%29.doc
http://www.freewebs.com/altara2s/Biology%20Chapter%202%20Notes%20%28Simplified%29.doc
http://www.freewebs.com/altara2s/Biology%20Chapter%203%20Notes.doc
http://www.freewebs.com/altara2s/Biology%20Chapter%204%20Notes.doc
http://www.freewebs.com/altara2s/Biology%20Chapter%205.doc
http://www.freewebs.com/altara2s/Biology%20Chapter%206%20Notes.doc
http://www.freewebs.com/altara2s/Biology%20Chapter%208%20Notes.doc
http://www.freewebs.com/altara2s/Biology%20Chapter%209%20Notes.doc
http://www.freewebs.com/altara2s/Biology%20Chapter%2013%20Notes.doc
http://www.freewebs.com/altara2s/Biology%20Chapter%2019%20Notes.doc
http://www.freewebs.com/altara2s/Biology%20Chapter%2024%20and%2025%20Notes.doc
Web site link: http://altara2s.webs.com/
Google key word : Biology notes and summaries file type : doc
Author : not indicated on the source document of the above text
If you are the author of the text above and you not agree to share your knowledge for teaching, research, scholarship (for fair use as indicated in the United States copyrigh low) please send us an e-mail and we will remove your text quickly.
Biology notes and summaries
If you want to quickly find the pages about a particular topic as Biology notes and summaries use the following search engine:
Biology notes and summaries
Please visit our home page
Larapedia.com Terms of service and privacy page
