Aluminium
Aluminium
The summaries, quotes and text on this page are used only for illustrative educational and scientific purposes and are free provided to users.
It is decorative. It is easily formed, machined, and cast. Alloys with small amounts of copper, magnesium, silicon, manganese, and other elements have very useful properties.
Strength depends on purity. 99.996 per cent pure aluminium has a tensile strength of about 49 megapascals (MPa), rising to 700 MPa following alloying and suitable heat treatment.
Although not found free in nature, Aluminium is an abundant element in the earth's crust.
A key property is low density. Aluminium is only one-third the weight of steel.
Aluminium and most of its alloys are highly resistant to most forms of corrosion. The metal's natural coating of aluminium oxide provides a highly effective barrier to the ravages of air, temperature, moisture and chemical attack.
Aluminium is a superb conductor of electricity. This property allied with other intrinsic qualities has ensured the replacement of copper by aluminium in many situations.
Aluminium is non-magnetic and non-combustible, properties invaluable in advanced industries such as electronics or in offshore structures.
Aluminium is non-toxic and impervious, qualities that have established its use in the food and packaging industries since the earliest times.
Other valuable properties include high reflectivity, heat barrier properties and heat conduction. The metal is malleable and easily worked by the common manufacturing and shaping processes.
Physical Properties
Density / Specific Gravity (g.cm-3 at 20 °C) |
2.70 |
Melting Point (°C) |
660 |
Specific heat at 100 °C, cal.g-1K-1 (Jkg-1K-1) |
0.2241 (938) |
Latent heat of fusion, cal.g-1 (kJ.kg-1) |
94.7 (397.0) |
Electrical conductivity at 20°C |
64.94 |
Thermal conductivity (cal.sec-1cm-1K-1) |
0.5 |
Thermal emmisivity at 100°F (%) |
3.0 |
Reflectivity for light, tungsten filament (%) |
90.0 |
These properties can be very significantly altered with the addition of small amounts of alloying materials. Aluminium reacts with oxygen to form a microscopic (0.000000635cm) protective film of oxide, which prevents corrosion.
Aluminium in massive form is non-flammable. Finely divided particles will burn. Carbon monoxide or dioxide, aluminum oxide and water will be emitted. This is a useful property for making rocket fuel.
ALUMINIUM This page starts by looking at the extraction of aluminium from its ore, bauxite, including some economic and environmental issues. It finishes by looking at some uses of aluminium. Extracting aluminium from bauxite Aluminium ore |
|||||||||||
|
Note: Bauxite actually contains one of a variety of hydrated aluminium oxides some of which you can write as Al2O3,xH2O. Since this is in itself a simplification, for UK A level purposes we normally just treat it as impure Al2O3. |
||||||||||
Purifiying the aluminium oxide - the Bayer Process |
|||||||||||
|
Note: You may find all sorts of other formulae given for the product from this reaction. These range from NaAlO2 (which is a dehydrated form of the one in the equation) to Na3Al(OH)6 (which is a different product altogether). |
||||||||||
The impurities in the bauxite remain as solids. For example, the other metal oxides present tend not to react with the sodium hydroxide solution and so remain unchanged. Some of the silicon dioxide reacts, but goes on to form a sodium aluminosilicate which precipitates out. Precipitation of aluminium hydroxide |
|||||||||||
|
Note: This all starts to be a bit of a nightmare if you try to get at the truth of what is happening. There are two separate issues here - both glossed over by most sources. If you like your life to be simple, ignore the rest of this note! |
||||||||||
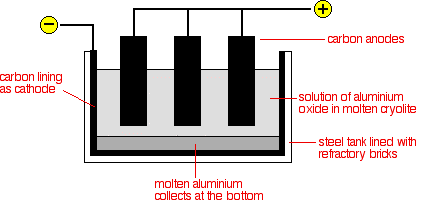
Formation of pure aluminium oxide Conversion of the aluminium oxide into aluminium by electrolysis The electrolysis cell
Although the carbon lining of the cell is labelled as the cathode, the effective cathode is mainly the molten aluminium that forms on the bottom of the cell. The electrode reactions
|
|||||||||||
|
Note: That all seems fairly obvious, and until I was doing the research for this page, I thought it was fairly obvious too. It's a pity it turns out to be wrong! It is currently believed that neither cathode nor anode reaction happens like this. There is a reaction between the aluminium oxide and the cryolite to produce a range of complex ions involving aluminium, oxygen and/or fluorine. It is the various complexes present which gain or lose electrons at the electrodes, rearranging themselves again in the process. I'm not giving details of this because I understand that there is still some uncertainty involved. |
||||||||||
Some economic and environmental considerations
|
|||||||||||
|
Note: This is deliberately brief because a lot of it is just common sense, and you will probably already have met it in detail in earlier chemistry courses, in geography, in general studies, or wherever. |
||||||||||
Economic considerations
Environmental problems in mining and transporting the bauxite
Extracting aluminium from the bauxite
Recycling
Uses of aluminium
Anodising essentially involves etching the aluminium with sodium hydroxide solution to remove the existing oxide layer, and then making the aluminium article the anode in an electrolysis of dilute sulphuric acid. The oxygen given of at the anode reacts with the aluminium surface, to build up a film of oxide up to about 0.02 mm thick. Some uses include:
|
|||||||||||
http://www.almolink.org/notes/aluminium.doc
Many thanks to Author : Alrick Moodie www.almolink.org/
Aluminium is the most abundant metal in the Earth's crust. Bauxite, the main ore of aluminium, is an impure form of aluminium oxide. To make aluminium, purified aluminium oxide (Al2O3) is dissolved in molten cryolite (Na3AlF6) at 900°C and then electrolysed.
Research about aluminium recycling
Quotes form source: http://www.mpma.org.uk
- Recycling one kilogram of aluminium can save up to 8 kilograms of bauxite, four kilograms of chemical products and 14 kilowatt hours of electricity.
- Anything made of aluminium can be recycled repeatedly: not only cans, but aluminium foil, plates and pie molds, window frames, garden furniture and automotive components are melted down and used to make the same products again.
- Used aluminium cans can be recycled to make new aluminium cans, aluminium windows can be recycled to make new aluminium windows and old aluminium engine blocks to make new ones.
- The recycling rate for aluminium cans is already above 70% in some countries.
- The aluminium industry has set up various schemes to encourage recycling in many countries.
- Aluminium beverage cans can be profitably recycled by individuals and groups and most countries have a national can recycling association which offers advice, support, and can put collectors in touch with purchasing organisations.
- Process scrap at all stages is meticulously collected and sorted by alloy by all aluminium companies and most customer organisations. Unlike other metals, scrap aluminium has significant value and commands good market prices.
- The London Metal exchange quotes aluminium scrap prices.
- Aluminium companies have invested in dedicated state of the art secondary metal processing plants to recycle aluminium. In the case of beverage cans, the process uses gas collected from burning off the volatile substances in can coatings to provide heat for the process. Every last bit of energy is used.
- Used beverage cans are normally back on supermarket shelves as new beverage cans in 6-8 weeks in those countries which have dedicated can collecting and recycling schemes.
- In Europe, the aluminium beverage can meets the minimum targets set in the European directive on Packaging and Waste. Sweden (92 per cent) and Switzerland (88 per cent) are the European can recycling champions. The European average is 40 per cent, a ten per cent increase since 1994.
- The recycling of aluminium beverage cans eliminates waste. It saves energy, conserves natural resources, reduces use of city landfills and provides added revenue for recyclers, charities and local town government. The aluminium can is therefore good news for the environment and good for the economy.
- The aluminium can is 100% recyclable; there are no labels or covers to be removed.
- Today's aluminium can requires about 40% less metal than the can made 25 years ago; hence the need for less energy and less raw material per can.
- Cans made from aluminium are worth 6 to 20 times more than any other used packaging material.
- Aluminium is the only packaging material that more than covers the cost of its own collection and processing at recycling centres.
Source: http://www.mpma.org.uk/pages/userdata/mpma/AluminiumRecyclingFact1.doc
Author: was not indicated in the source document
Aluminium
Visita la nostra pagina principale
Aluminium
Disclaimer
This site is not a news organization and is updated without any periodicity, solely on the basis of availability of material, so is not an editorial product subject to discipline in art. 1, paragraph III of Law No. 62, 7.03.2001. The summaries, notes, lyrics and quotes contained on this site are available free of charge to students, researchers, professors, technicians with illustrative educational and scientific purposes with the concept of fair use and purpose of compliance with EU Directive 2001/29 / EC and the Law Article 633. Dlg 70 and 68. The site is managed and coordinated by the author only for informational and educational purposes. While considering the reliable sources used, the author of this site does not guarantee the accuracy and integrity of information and therefore accepts no responsibility for any problems or damage caused by errors or omissions, if such errors or omissions result from negligence , accident or other cause. All notes, quotes the texts and images are property of their respective authors and production companies that own the rights, if the beneficiaries were considered damaged by the inclusion of these files on this site or had been inadvertently inserted images, information, text or other copyrighted material will be immediately removed and or it will be referred to the source from simple message to the e-mail address indicated on the contact page.
The mission of this site is the progress of science and useful arts, as we think they are very important for our country's social and cultural benefits of the free sharing of information. All information and images on this site are used here only for educational purposes, cognitive and informative. The medicine and health information contained on this site is general in nature and informative purposes only and therefore can not replace in any case the advice of a doctor (or a legally authorized person to the profession). On this site we have made every effort to ensure the accuracy of tools, calculators and information, we can not give a guarantee or be held responsible for any errors that were made, the texts used were taken from sites that have put them in available free of charge to make them known on the web with educational purposes. If you find an error on this site or if you find a text or tool that may violate any applicable laws of copyright, please notify us via e-mail and we will promptly remove it.