Corrosion
Corrosion
The following texts are the property of their respective authors and we thank them for giving us the opportunity to share for free to students, teachers and users of the Web their texts will used only for illustrative educational and scientific purposes only.
The information of medicine and health contained in the site are of a general nature and purpose which is purely informative and for this reason may not replace in any case, the council of a doctor or a qualified entity legally to the profession.
![]()
Corrosion
Chemistry
Corrosion
Leave a piece of shiny metal outside for a while and it soon changes colour - it becomes tarnished. A new car without care will soon lose its newness. Stonework becomes disfigured and discoloured. The atmosphere reacts with these materials causing them to deteriorate. Three main areas of concern are water mains and sewage pipes, motor vehicles and defence. The costs are enormous - not only for direct damage-but also in indirect costs for lost time and productivity due to outages, delays, failure and litigation. It has been estimated that at $2.8 trillion, the annual cost of corrosion world-wide is over 3% of the world's GNP.
Oxygen and water are the chief reactants, but other substances such as sulfur dioxide, SO2, and nitrogen dioxide, NO2 are also involved. As with other chemical reactions, an increase in temperature causes an increase in the rate of deterioration.
The chemical decomposition of solid materials made by humans is called corrosion. Corrosion is defined as the unintentional destruction of a solid usually by redox, by reaction with the atmosphere, or whatever the surrounding environment might be - starting at the surface. The term corrosion is mainly used to denote the reaction of metals and alloys when they are exposed to nature. The rusting of iron is the most common example.
Because corrosion is so costly, it is important to know how to prevent it. For this it is necessary to first learn about the chemistry of corrosion. The chemistry of corrosion is galvanic as you will soon discover.
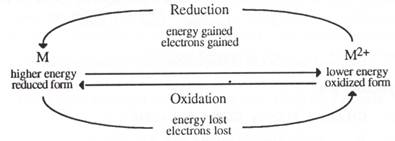
Most metals occur in nature in combined form such as oxides, hydroxides, chlorides, carbonates, sulfates sulfides and silicates. The extraction of metals from their ores requires a considerable amount of energy. The isolated metals can be regarded as being in a much higher energy state than their corresponding minerals and will show a natural tendency to return to their lower energy or combined state. Thus corrosion can be thought of the reverse process to that of extracting them from their ores. The extraction process involves reduction.
 .
.
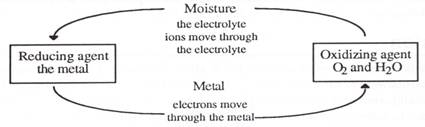
Corrosion is a redox reaction. In addition, metals allow the easy passage of electrons through their lattices. If moisture is present, this can act as an electrolyte. We now have all the requirements for a galvanic cell.
Factors influencing corrosion
What conditions or factors influence of help corrosion? If we know what helps corrosion, we are in a better position to stop it. The factors are:
● the reactivity of the metal
● the presence of a cathodic metal
● the effect of oxygen, moisture and dissolved ions
● the nature of the oxide formed on the metal surface
● the effect of temperature
● the effect of acids
Reactivity of the metal
The more reactive the metal, the more readily it will enter into chemical reaction, and the more readily it will corrode. The least active metals do not corrode because they do not react. King Tutankhamen, the boy Pharoah who died in about 1350 BC was entombed in a corrosion-proof coffin made of solid gold. It weighs about 1111 kg and is just as shiny today as it was over 3000 years ago.
The metals at the top of the metal activity series are called anodic metals; anodic because they are easily oxidised. The word anode is used to describe a location where oxidation occurs in an electrochemical cell. Anodic metals corrode readily because they lose electrons readily.
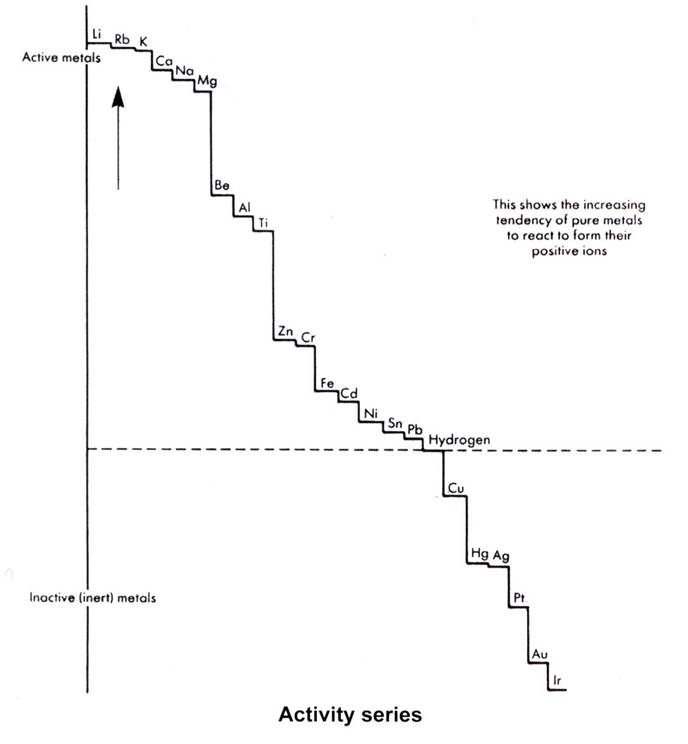
Cathodic metals are those at the bottom of the metal activity series. The cathode is a location where reduction occurs in an electrochemical cell, and so in a galvanic couple, where there are just two different metals, the cathodic metal is the less reactive of the two. This term is used therefore in two ways, but in both usages, cathodic metals, unlike anodic metals, are unreactive. These metals are also called noble metals because of their inability to react. The diagram on the next page of a metal reactivity series gives a scaled comparison of some metals' tendency to lose electrons. Hydrogen is included as a reference. The metals above hydrogen react with dilute acids to produce hydrogen; those below do not. The scale is based on a numerical one under standard conditions to give an indication of reactivity or lack of reactivity. The reactions for comparing reactivity are, in general:
M → M+ + e –
M → M2+ + 2e –
where M stands for any metal.
For general chemistry purposes these metals are placed into three groups:
● the most reactive: K, Na, Ca, and Mg. These all react with cold water.
● the reactive: Zn, Ni, Fe, Sn and Pb are in the middle, and react with dilute acids, not cold water.
● the least reactive: Cu, Hg, Ag, Au and Pt are at the bottom, below H2, and do not react with dilute
acids or water.
Most metals in this last group can be found uncombined in nature, a visual proof of their reactivity.
A galvanic series
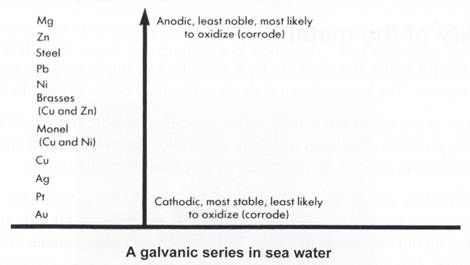
But this table is of limited use under environmental conditions. For one thing, only metals and not alloys are included. Most metals are alloyed for use. Secondly, the table was devised under standard conditions where each pure metal is in a 1.0 mol L-1 solution of its ions to form a redox pair. One is unlikely to find copper in a 1.0 mol L-1 solution of copper sulfate in nature! Chemical engineers prefer to use a table called 'the galvanic series' and one is shown for some more important industrial metals. This series was determined in sea water, a fairly common environment for some metals, e.g. the steel hull of a ship.
Presence of a cathodic metal
Corrosion always occurs most readily when two dissimilar metals are joined in a conducting solution, called an electrolyte. A galvanic cell forms. The second, more inert metal bleeds electrons from the anodic metal and so a continuous flow of electrons occurs if the circuit is completed. Oxidation of the anodic metal occurs and oxidation means corrosion.
The rate of corrosion does depend upon which metals are joined, When copper is present, the rate of corrosion of iron may increase by as much as 1000 times.
In the 18th century, the HMS Alarm was a British Royal Navy ship, the first to bear this name, and the first to have a copper-sheathed hull as an experiment to reduce woodworm attack and barnacle growth. However, it was discovered that after several years the sheathing had become detached from the hull in many places because the iron nails used to attach the copper to the timber had become severely corroded in sea water. Thus copper sheathing was removed from Alarm and several other test vessels. The results could have been 'alarming'! The Statue of Liberty in New York harbor, the biggest metal statue in the world, was a gift from the French people to the American people in the late nineteenth century. It needed extensive restoration when it was discovered that the asbestos soaked cloth designed to insulate the copper skin from the iron framework had become porous and soaked up salt water like a wick. This provided an electrolyte. The iron framework was interconnected and so only needed one point of contact with the copper skin and electrolyte for accelerated galvanic corrosion of the anodic iron bars to occur. Some lost up to two thirds of their cross-sectional area. The expansive force of the rust formed ripped out rivets leaving gaping holes. All the iron bars had to be replaced and repairs were made to the copper skin to stop it leaking more sea water.
Can you imagine what might happen if a mercury thermometer were to break inside an aluminium container? This used to be a big problem in breweries. Many impure metals corrode rapidly because the impurities are cathodic metals. In the presence of an electrolyte, these form galvanic cells. The anodic metal is eaten away, and the result is pitting.
Effect of oxygen, moisture and dissolved ions
Active metals do react directly when in contact with oxygen to form an oxide layer. The very active metals such as sodium and potassium must be kept under oil in order to prevent such rapid corrosion, that fire could result.
With aluminium, chromium, nickel, titanium and zinc, the surface oxidation, although rapid, prevents further oxidation by sealing the surface tightly with a water insoluble oxide layer.
But it is the combination of oxygen, moisture and dissolved ions to make an electrolyte which is the most damaging. The effect of this combination, particularly on the corrosion of iron is easily demonstrated.
This experiment demonstrates three more factors important in corrosion.
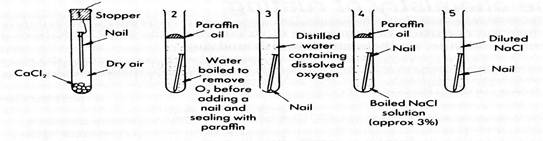
● the presence of air or oxygen
● the presence of water
● the presence of dissolved ions in the water
Nature of the oxide formed on the metal surface
If aluminium is used instead of iron, little evidence of corrosion is detected. The reason for this is that aluminium forms a self-sealing, impervious oxide layer which is able to stick tightly to the metal. It is also so thin, it is invisible. Aluminium retains its metallic sheen. This Al2O3 is one of the hardest substances known. It is harder than the metal itself and can be thickened for additional protection by anodising. Chromium is another metal that forms a self-sealing oxide layer which in turn prevents further corrosion. Iron also forms an oxide layer called rust, but this layer is porous and crumbly, continually exposing the metal to further attack. Thus although aluminium is much higher on the metal reactivity series than iron, it is resistant to destructive corrosion. The nature of the oxide formed during corrosion also determines the extent of corrosion.
Effect of temperature
In a hot, dry atmosphere, the rate of corrosion is very low indeed. There is simply not enough electrolyte present. The water has evaporated. However, in very cold areas, such as Canada and Northern Sweden, corrosion is also very slow. The freezing climate removes atmospheric moisture as ice. Corrosion rates, like so many other chemical reactions, are affected by temperature. Everything else being equal, metals corrode faster at higher temperatures than lower ones.
Effect of acids
Dilute acids attack iron very rapidly. The reaction is:
Fe(s) + 2H+(aq) → H2(g) + Fe2+(aq)
Any acid in the atmosphere will increase corrosion by direct action. Furthermore, acidic solutions are good electrolytes and encourage electrochemical attack as well.
All rain water is acidic because it absorbs carbon dioxide, but industrial gases such as sulfur dioxide can increase the acidity of rain considerably.
The first appearance of acid rain with an acidity exceeding that of clean rain seems to have coincided with the onset of the Industrial Revolution in the mid-19th century.
Not only metal structures are at risk from acid rain. Stone structures, such as buildings and monuments are also attacked by acid rain. |
|
The chemistry of rusting
Iron is the Earth's most abundant element because the 7000 kilometre diameter core of the planet consists mainly of the molten metal. In the Earth's crust it is present in about 4.1% and is the second most abundant metal after aluminium - 8.2%. However, iron is far easier to extract than aluminium, is cheaper than aluminium, has more applications than any other metal and accounts for about 90% of all metal that is refined. Rust is the problem. Because the corrosion of iron is the most common, the most expensive and the most annoying, its chemistry is worth studying.
Rust is a hydrated iron(III) oxide, Fe2O3.xH2O, and is the result of redox reactions between iron, water and oxygen. Iron is oxidised and eaten away and holes form. The reactions are:
Fe(s) → Fe2+(aq) + 2e –
Fe2+(aq) → Fe3+(aq) + e –
Overall Fe(s) → Fe3+(aq) + 3e –
Oxygen and water are reduced using the electrons supplied by the iron:
2H2O + O2(g) + 4e – → 4OH –(aq)
The hydroxide ions and iron(III) ions combine to form iron(III) hydroxide:
Fe3+(aq) + 3OH –(aq) → Fe(OH)3(s)
This reacts with more water to form rust, which is Fe2O3.xH2O(s)
Rust is porous and permeable and exposes more metal to the oxygen as it flakes away. Thus rusting will continue.
A further complication with the rusting of iron is that a galvanic cell can form on the one metal. This is due to differences in dissolved oxygen concentration. The area high in oxygen concentration, the circumference of the droplet, becomes the cathode, the area low in oxygen concentration at the centre, the anode. Rusting on the surface of iron, in a water droplet, or a drop of salt solution, can be represented diagrammatically as shown.
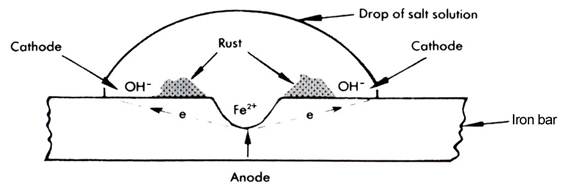
The chemistry is the same as given above with oxidation of iron occurring at the anode and reduction of oxygen at the cathode. When the Fe3+ ions migrate to the cathode and OH – ions migrate to the anode, they meet between the electrodes forming a ring of rust.
Prevention of corrosion
From the chemistry given, the protective measures to prevent corrosion are easy to understand. However, they are still expensive and time consuming. Preventative measures include:
● covering the surface ● using a sacrificial anode ● cathodic protection ● good design ● control of environmental conditions
Covering the surface Corrosion is a reaction that starts at the surface. Therefore to cover the surface to prevent contact with oxygen and moisture and contact with a more cathodic metal is the most obvious preventative measure.
Protective surfaces are widely used to prevent corrosion and include: |
|
● paint, cement, bitumen, PVC, fibreglass, PVC, rubber or even the carport or a tarpaulin.
Metal objects must be corrosion free before painting, otherwise corrosion will continue. Rust is of
larger volume than iron and lifts off paint coatings. Removal of rust and soluble salts is expensive and
difficult, particularly on structures that have already been erected such as pylons and bridges. Sand
blasting and flame blasting are the most effective. Repainting is best done before damage has taken
place to the primer coat.
● natural protection provided by stable, impermeable and adherent oxide or carbonate coatings.
Chromium, titanium and zinc form oxide coatings. Aluminium, an active metal, is rendered non-
reactive by its aluminium oxide coat and this can be thickened by the electrolytic process called
anodising. Zinc's oxide coat is slowly converted to the carbonate, which is also protective
Bronze becomes coated with red copper(I) oxide and a green copper carbonate. The
bronze cover, called 'patina', is both decorative and protective.
● alloying iron with chromium to form stainless steels. The chromium forms a self-repairing Cr2O3
layer.
Electroplating
This is the most important of all methods of applying metal coatings to base surfaces. It is used:
|
● to provide protection from corrosion. Nickel, tin, zinc, lead, chromium, and gold are used. 'Tin' cans are plated with tin.
● to beautify an unattractive metal finish. Gold, silver and chromium are used and a coating of these is cheaper and the final product stronger, than if say, gold were used throughout.
● To provide an undercoating for another metal to adhere to. Iron and its alloys are frequently electroplated with copper and nickel before the final coating of chromium is electrolytically applied. Silver is usually plated onto copper. Copper sticks well to iron, and chromium and silver stick well to copper - better than they do to iron.
Electroplating reduces cost because very thin coatings can be applied. |
The general method is to use an electrolytic cell. The metal object to be plated is the cathode. The electrolyte contains ions of the metal to be plated out.
The anode is composed of the metal being plated out at the cathode. Its function is to replenish ions lost from the electrolyte.
For the electroplating of copper, the reactions are:
At the anode Cu(s) → Cu2+(aq) + 2e –
replenish Cu2+ ions
lost at the cathode
At the cathode: Cu2+(aq) + 2e – → Cu(s)
The colour of the electrolyte remains constant.
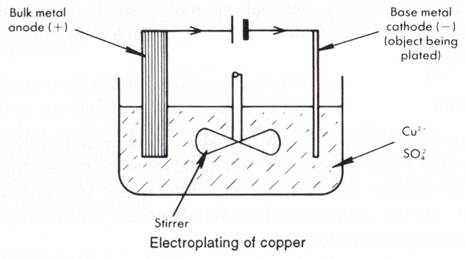
The most crucial factor to make sure the metal coating sticks well and is dense and not spongy, is the nature of the electrolyte, but concentration, temperature, pH and current density are also important and determined by extensive experimentation. For example, during the electroplating of copper, pin holes may appear in the copper at the cathode due to the release of H2.
2H+(aq) + 2e – → H2(g)
This is caused by having to much H+(aq) present in the solution. The pH is too low!
Using a sacrificial anode
This converts the metal to be preserved, usually iron, from an anode to a cathode. Cathodes do not react!
Some examples include:
● Magnesium anodes are used to protect a ship's hull which is the cathode. They are designed to last
the time from refit to refit of the ship.
● Underground pipes are protected both inside and out by connecting them at intervals to 'sacrificial
anodes' of magnesium. The electrons which flow from magnesium to iron, suppress the iron's
tendency to lose electrons and be oxidised.
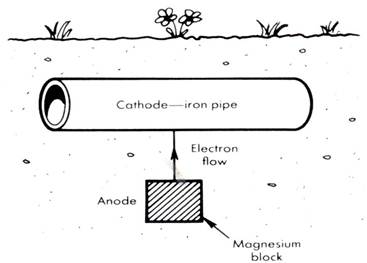
At the magnesium anode: Mg(s) → Mg2+(aq) + 2e –
At the iron cathode: 2H2O + O2(g) + 4e – → 4OH –(aq)
Simply stated, the magnesium is sacrificed instead of the iron
● Galvanised iron is iron given protection by a coating of zinc. This is called passive
protection - just like a coat of paint or plastic. However, the iron is protected even when the zinc is
scratched, and the surface is exposed. The zinc, although rendered non-active by its oxide-carbonate
coat, is still anodic to iron, and is eaten away instead of iron. This is active protection.
At the zinc anode: Zn(s) → Zn2+(aq) + 2e –
At the iron cathode: 2H2O + O2(g) + 4e – → 4OH –(aq)
Like the magnesium in the first example, the zinc being more active than iron, reacts instead of the iron.
Zincalume steel which is more lightweight than galvanised iron and is even more corrosion resistant has largely replaced it as a roofing material. lt also offers both passive and active protection.
Sacrificial anodes must be periodically replaced. They can only be used where there are low levels of
electrical activity. Although the metal used is more expensive than the iron it protects, sacrificial anodes are cheaper to replace than say, a long segment of underground steel pipe.
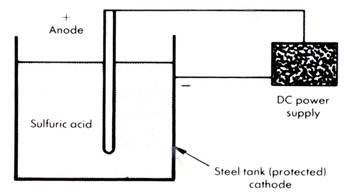
Cathodic protection
This is used where there is likely to be a high level of electrical activity. A direct power source provides a flow of electrons. The metal receiving the electrons becomes the cathode and will not corrode. The negative end of the power source is connected to the protected structure and the positive end to a set of anodes, which completes the circuit. The anodes must be replaced periodically.
In this example the steel tank is prevented from corroding even though it is in an acid environment.
Without cathodic protection, the acid would corrode the tank and regular and expensive replacements would be necessary.
|
|
Design
Good design can prevent corrosion. Dissimilar metals should be insulated from each other to prevent a flow of current. Steel objects need to be designed in such a way that moisture is never trapped and can readily drain or evaporate.
The car has a number of areas where design may prevent exclusion of moisture. Which parts rust?
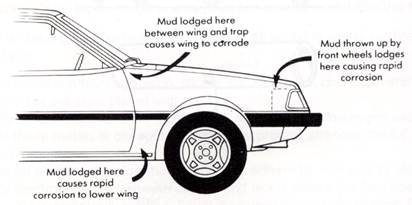
Moisture exclusion is important even when paint and rubber coatings are used as these are very slightly porous and cannot be relied upon to keep out oxygen and moisture. Once corrosion starts, even to a very slight extent, the lift off of the surface coating material by rust soon increases the rate of corrosion.
New plastics may offer an increasing range of alternate materials to metal. A plastic exhaust pipe that has a very high melting point would seem a sensible replacement for the mild steel ones used at present. These are eaten away very rapidly by acidic exhaust gases. The number of firms offering replacement exhaust pipes, show how serious this problem is.
Replacement of metal parts with plastic, offers the additional benefit of a lighter vehicle and so a greater fuel economy.
Control of environmental conditions
The emission of sulfur dioxide from industrial complexes has produced a corrosion problem on a large scale.
Sulfur dioxide and other acidic oxides dissolve in water to produce 'acid rain'. Reliable studies show that the average precipitation in the Northern Hemisphere is a hundred times more acidic than 250 years ago. This problem, which is a serious one, could be reduced if more of the sulfur dioxide was captured to make sulfuric acid. Flue gas desulfurisation is another solution where the waste gases are passed through a slurry of lime to remove sulfur dioxide before it reaches the top of a smoke stack. These installations are called 'scrubbers'. Pollution control is corrosion control.
Conclusion
The more chemists study corrosion, the more they realise that it is a very complex problem. Two facts stand out. The first is that corrosion is a natural process and will occur if electron movement is at all possible. The second is that corrosion can be controlled, and avoided if appropriate measures are taken. Although expensive, preventative measures are cheaper than replacing materials damaged by corrosion.
Exercises
1(a) What is corrosion?
(b) Why is CO2 a factor in speeding up corrosion?
(c) What is meant by a galvanic cell?
(d) What is an anodic metal and what is a cathodic metal?
(e) 'Corrosion can be used to prevent corrosion', Give two examples where the above statement applies.
(f) A lot of corrosion could be prevented by careful design. Give specific examples, and discuss as fully
as possible.
2(a) Why is rain water always acidic? (Include two equations in your answer).
(b) What other substance(s) may be present in the air to increase the acidity of rain water?
(c) Explain why an acidic solution can speed up the corrosion of iron.
(d) Corrosion is more prevalent by the sea and yet dissolved calcium chloride may actually slow down
corrosion. Can you explain both these observations?
(e) Explain why steel objects which have lain at the bottom of the sea for decades (or even centuries),
show very little sign of corrosion.
3 For the following questions, choose the best way to prevent corrosion. There may be more than one
correct answer.
● Paint it
● Leave it exposed to air because it forms its own protective coating.
● Leave it as it is because it does not corrode.
● Coat it with another metal.
(a) Steel used to make cans for food. (b) Gold filling for a tooth. (c) Copper to be used for water pipes. |
(d) Platinum used to make a ring (e) Steel to be used as roofing material (f) Steel to be used in making a motor car body. |
4 An experiment is set up as shown below in three petrie dishes with the metal objects immersed in
dilute NaCl(aq).
(a) In which case is corrosion of iron fastest, which slowest and why?

(b) Zincalume steel is a common material used for roofing and provides both passive and active
corrosion protection. Can you explain why in the installation instructions it is stated, 'incompatible
with lead flashing and copper piping'?
5 Explain using a labeled diagram, how cathodic protection can be used to suppress corrosion of a steel
tank used to store acid. Draw a sketch diagram, label the anode and the cathode and show the direction
in which electrons move. What is the sign of the anode?
6 Rapid corrosion can occur in iron storage tanks even if a few drops of water separate at the bottom.
Both the oil and water contain dissolved oxygen.
(a) What is the formula of rust? Where will it form?
(b) Why will the reaction occur so rapidly?
(c) Give the equations for rust formation and suggest two methods to prevent the formation of rust in
steel storage tanks.
7 Calcium hydroxide is used in scrubbers in industry to remove SO3 (and SO2) from waste gases from
the burning of fossil fuels. Calcium sulfate, CaSO4.2H2O (called gypsum) is formed.
(a) What is 'acid rain'? Suggest how it forms from SO3(g).
(b) Describe using equations where possible, what could happen to the following objects in 'acid rain'.
(i) a marble statue
(ii) a steel pylon
(iii) a cotton umbrella
(c) It is claimed that every tonne (1000 kg) of sulfur in SO2 produces 5 tonne of gypsum and this in
itself provides a disposal problem.
(i) See if you can confirm these figures with a simple calculation.
(ii) Can you suggest ways of using SO2, SO3 or gypsum to overcome these problems?
8 Iron rusts in the presence of oxygen and water.
(a) Write balanced equations for:
Fe → Fe3+
O2 → OH –
(b) Which species is the reducing agent? Why?
(c) What is the oxidation number of iron in Fe2O3?
(d) Name one method used to prevent the rusting of iron.
9 On page 4 of this chapter, the Statue of Liberty is given as an example of where galvanic corrosion
occurred, causing enormous damage.
(a) Why was there only mechanical damage done to the copper skin?
(b) The two dissimilar metals were insulated from each other. Why then did galvanic damage occur?
(c) The iron bars making up the inner framework were so corroded, they had to be replaced. Can you
suggest what a suitable replacement might be? Remember that the Statue of Liberty is a colossus
and constantly buffeted by strong winds, so plastics would not be suitable.
Source : http://www.usc.adelaide.edu.au/local/transitionlectures/chemistry/corrosion.doc
Web site link: http://www.usc.adelaide.edu.au/
Google key word : Corrosion file type : doc
Author : not indicated on the source document of the above text
If you are the author of the text above and you not agree to share your knowledge for teaching, research, scholarship (for fair use as indicated in the United States copyrigh low) please send us an e-mail and we will remove your text quickly.
Corrosion
If you want to quickly find the pages about a particular topic as Corrosion use the following search engine:
Chemistry
Corrosion
Please visit our home page
Larapedia.com Terms of service and privacy page