Elements, Compounds and Mixtures
Elements, Compounds and Mixtures
The following texts are the property of their respective authors and we thank them for giving us the opportunity to share for free to students, teachers and users of the Web their texts will used only for illustrative educational and scientific purposes only.
The information of medicine and health contained in the site are of a general nature and purpose which is purely informative and for this reason may not replace in any case, the council of a doctor or a qualified entity legally to the profession.
![]()
Elements, Compounds and Mixtures
Chemistry
Elements
The different types of atom are arranged in terms of their size in a table called the Periodic Table of elements.
Elements are substances which are made up of only one type of atom
Molecules
Some atoms form a very strong attachment (‘bond’) to another atom or atoms and as a result they always go around in groups of two or more atoms. We call these guys ‘molecules’.
It is very hard to break these ‘bonds’ and we say that in this case the atoms are ‘chemically combined’. We will look at this bonding in detail in another chapter later.

Basically a molecule is like a very small group of atoms that go around together (they are still too small to see).
Examples:
For the element oxygen the atoms go around in pairs of oxygen atoms.
These are called oxygen molecules and that is why oxygen is often represented as O2.
For the compound water the atoms go around in ‘gangs’ consisting of two hydrogen atoms and one oxygen atom. These ‘gangs’ are called water molecules and that is why water is often represented as H2O.
Compounds
A compound is formed when two or more atoms of different elements combine together chemically
My head hurts; what’s the difference between a compound and a molecule?
All compounds are molecules, but not all molecules are compounds (e.g. H2 is a molecule because it is composed of two atoms chemically combined, but because they are both hydrogen atoms the molecule is not a compound).
Remember when we said that atoms are so small that you can’t see them?
Well molecules are made up of small groups of atoms so you won’t be able to see them either.
A compound however might be something like table-salt; you can hold it in your hand. It is a compound because it is made up of two different types of atom – in this case sodium and chlorine. The chemical name for table-salt is NaCl.
The interesting thing is that both sodium and chlorine can be fatal if ingested (swallowed) but when the two go together to form table salt the result is perfectly safe (once you don’t eat too much!).
We can summarise this as follows:
When elements combine to form compounds they may lose their individual properties.
Other examples of compounds and their constituent elements
Compound |
Symbol |
State of matter (at room temp) |
Elements in the compound (and state at room temp) |
Water |
H2O |
liquid |
Hydrogen (gas) oxygen (gas) |
Carbon dioxide |
CO2 |
gas |
Carbon (solid) oxygen (gas) |
Magnesium oxide |
MgO |
solid |
Magnesium (solid) oxygen (gas) |
Iron sulphide |
FeS |
solid |
Iron (solid) Sulphur (solid) |
Mixtures
If a substance is made up of different components but they are just mingled together rather than combined at an atomic level then we call this a mixture.
A mixture contains two or more different substances mingled together but not chemically combined
Mixtures can be (fairly) easily separated, whereas compounds cannot be easily separated.
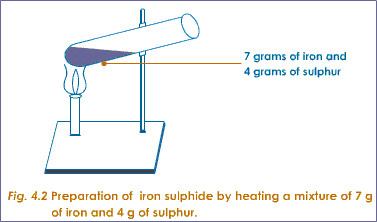
For example if you pour sulphur power over iron filings and mix them together you get a mixture, and to separate them simply use a magnet which will attract the iron filings and leave the sulphur powder behind.
However if you heated the mixture to a very high temperature their chemical compositions will change and the two elements will ‘bond’ together chemically to become a new substance called iron sulphide. This cannot now be separated and the result is called a compound.
Source : http://www.thephysicsteacher.ie/JC%20Science/JC%20Chemistry/Student%20Notes/2.%20Elements,%20Compounds%20and%20Mixtures.doc
Web site link: http://www.thephysicsteacher.ie
Google key word : Elements, Compounds and Mixtures file type : doc
Author : not indicated on the source document of the above text
If you are the author of the text above and you not agree to share your knowledge for teaching, research, scholarship (for fair use as indicated in the United States copyrigh low) please send us an e-mail and we will remove your text quickly.
Elements, Compounds and Mixtures
If you want to quickly find the pages about a particular topic as Elements, Compounds and Mixtures use the following search engine:
Chemistry
Elements, Compounds and Mixtures
Please visit our home page
Larapedia.com Terms of service and privacy page
