Alcohols, Carboxylic Acids and Esters
Alcohols, Carboxylic Acids and Esters
The following texts are the property of their respective authors and we thank them for giving us the opportunity to share for free to students, teachers and users of the Web their texts will used only for illustrative educational and scientific purposes only.
The information of medicine and health contained in the site are of a general nature and purpose which is purely informative and for this reason may not replace in any case, the council of a doctor or a qualified entity legally to the profession.
![]()
Alcohols, Carboxylic Acids and Esters
Chemistry
Alcohols
Introduction
Alcohol is a familiar term to chemists and non-chemists alike. Methanol, also known as methyl alcohol, is used as an additive to unleaded petrol. Rubbing alcohol, propan-2-ol, is used to remove bacteria from the skin before surgery, or an injection. It is a bacteriocide and is also used for massages and as a base for perfumes and creams.
Ethyl alcohol is the main component in methylated spirits ('metho').
All of these alcohols and others, are used in the laboratory and chemical industry as solvents and reagents.
Some alcohols you may have heard of include menthol, found in peppermint oil and also made synthetically, is used in sore throat medicines and in creams for the relief of muscle aches; glycerol, also known as glycerine and is used in cosmetics, and cholesterol which can deposit in our arteries so causing heart problems.
To a non-chemist the alcohol is ethyl alcohol - the intoxicating part of beer, wines and spirits. It has, however, given its name to a family of compounds all of which have the same functional group. A chemist calls ethyl alcohol, ethanol.
Ethanol has the same carbon skeleton as the alkane ethane, and differs from it by having a hydrogen atom removed from one of the carbon atoms, and replaced by an _OH group, called hydroxy.
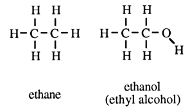
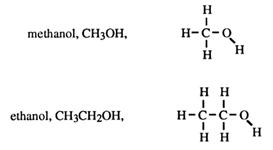
The observant reader will notice that the names of the alcohols given in this introduction end with -ol
Th e fu n cti o n al g ro u p -OH , hydroxyl, has had it ’s name updated to “hydroxy”.
(from Nomenclature of Organic Chemistry 1993, published by IUPAC).
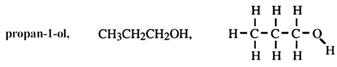
Naming alcohols
Alkane → alkanol. For example:
CH3OH is methanol
CH3CH2OH is ?
For longer chains, a position number of the hydroxy group is given. For example:

Regardless of branches, number from the end closer to the functional group.
Ethanol can be used as a fuel. It was used to extend petrol supplies during World War II. Petrol-alcohol mixes are called 'gasahol'. Brazil cultivates sugar cane on a large scale to produce fuel for trucks, cars and boats. It is cheaper in Brazil to buy a vehicle running on
100% ethanol than one using pet rol. T his reduces Brazil's dependenc e on oil- pr oduc ing coun tries and provi des a commercial outlet for local sugar cane growers.
The name fermentation comes from the Latin fermentum, to boil. The bubbles of CO2(g) give the mixture an appearance of boiling.
Can you write an equation for this reaction?
Preparation of ethanol
Ethanol is the most important member of the alcohol family. Its preparation by fermentation is so well known it has been referred to as 'the oldest chemical synthesis.' However, it is not important only because it is well known as a beverage. Ethanol is the most widely used industrial solvent. It is sold as methylated spirits, which is approximately 95% ethanol. The remainder contains poison - usually methanol - to make the ethanol undrinkable. Adding the poison cuts the cost, as alcoholic beverages are taxed by the government.
Fermentation
Some industrial alcohol is still made by yeast fermentation of sugar. Fermentation is the name given to the reaction that produces ethanol from sugars using yeasts. The yeasts produce the special catalysts, called enzymes, which cause the breakdown of the sugars.
The fermentation reaction for the sugar glucose is:
yeast
C6H12O6(aq) → 2C2H5OH(aq) + 2CO2(g)
glucose ethanol
More about fermentation is given in Biomolecules at the end of this chapter.
Addition of water to alkenes
Ethene from the catalytic cracking of gas oil can be converted to ethanol.
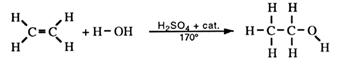
The alcohol produced this way is used as a solvent. Propene (propylene) is used to produce propan-2-ol (rubbing alcohol).
Physical properties of the alcohols
The first three alcohols are completely soluble in water. After this, solubility in water declines. Alcohols are soluble in other liquid alcohols and in other organic solvents.
Their boiling points and melting points are higher than those of the corresponding alkanes, for example there are no gaseous alcohols at room temperature. Methanol, the simplest alcohol, has a boiling point of 64.5°C. Methane boils at -161.5°C.
Chemical properties of the alcohols
Combustion
Alcohols burn with a clean blue flame to give carbon dioxide and water.
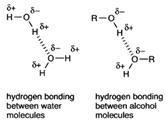
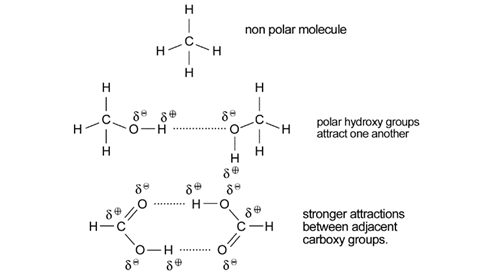
Hydrogen bonding is used to explain both the solubility of alcohols in water, and their much higher boiling points than the corresponding alkanes.
CH3OH(l) + 3_ O
2 2(g)
→ CO
Methanol and ethanol have a high octane number and can be used with petrol.
Oxidation
With air
Air bubbled through poor-quality wine produces vinegar. The reaction is speeded up by enzymes that are produced by certain bacteria.
enzyme
CH3CH2OH(aq) + O2 
in wine vinegar
(about 5 to dilute aqueous acetic
10% alcohol) acid (5 to 10%)
With acidified potassium dichromate
When ethanol is warmed with acidified potassium dichromate, the colour of the oxidant changes from orange to green. The half-equation for acidified potassium dichromate acting as an oxidant is:
Cr2O72— + 14H+ + 6e— → 2Cr3+ + 7H2O
orange green
Ethanol produces acetic acid again as it does with oxygen. All alcohols with no branch (i.e. methyl or ethyl group) at the point of attachment of the _OH group are oxidised by acidified potassium dichromate to the corresponding carboxylic acid.
The equation can also be written like this:
Cr2O7 2—/H+
2- +
The breathalyser
The simplest form of a breath analyser is used as a screening device. It contains potassium dichromate-sulfuric acid reagent absorbed in particles of silica gel
CH3CH2OH
Cr2O7 /H

CH3COOH
in a sealed glass ampoule.
The equation above, which focuses on organic reactants and products, is called an annotated equation. Organic chemists frequently use annotated equations, which are like one-step flow charts, as a useful simplification.
Reduction of alcohols with sodium
Alcohols can be seen to resemble water in structure. All three have a hydroxy group.
When alcohols react with sodium, hydrogen is generated, but less vigorously than with water. This is a test for alcohols.
Breath alcohol screening device
Alcoholic air blown through the tube causes a colour change, which gives an indication of blood alcohol content. More sophisticated meters are now used.
With water:
HOH + Na → ½ H2 + NaOH
HOCH3 + Na → ½ H2 + NaOCH3
sodium
methoxide
With methanol:
An ester is a compound formed between an alcohol and an organic acid.
Conc. sulfuric acid is a catalyst for making esters.
Malic acid (the most common carboxylic acid) occurs in apples, citric acid occurs in citrus fruit,
Alcohols react with acids to produce sweet smelling esters
Many esters are responsible for the odour and flavour in wines and fruit e.g. banana, peach, apple, pineapple and apricot. Some of these esters are made artificially and sold as fruit essences used in cooking. A word equation for the formation of esters is:

For example:
The reaction is called esterification.
Carboxylic acids
lactic acid occurs in sour milk, and
oxalic acid occurs in rhubarb.
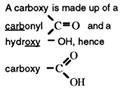
The functional group - COOH, carboxyl, has had its named updat ed t o carboxy (f rom Nomenclature of Organic Chemistry 1993, published by IUPAC. )
The chemists call the organic acids, carboxylic acids. The name comes from the functional group that contains a carboxy group.
Carboxylic acids are very common in nature. They are responsible for the sour taste of unripe fruits, and the taste in some ripe fruit such as lemons. Tartaric acid, found in grapes, is so called because its taste is sharp or tart.
Replacing a hydrogen atom in an alkane by a COOH group gives an alkanoic acid.

The carboxylic acids have a general formula, CnH2n+1COOH, and their functional group, _COOH, is called the carboxy group, or carboxylic acid group.
Naming carboxylic acids

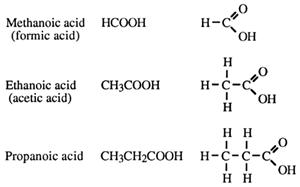
→ alkanoic acid. No position number for the carboxy group is needed as it is
Since the functional groups are the onl y p arts of the mol ecu les undergoing chemical change, it can be tedious to keep writing down the rest of the molecule which does not react.
Organic chemists frequently just write R as an abbreviation for the rest of the molecule. Hence, R-OH, R-COOH etc.
What is the systematic name of butyric acid, CH3CH2CH2COOH?
always at the beginning of the parent chain (and forms part of the parent chain).
Physical Properties of the carboxylic acids
They have a sharp taste and characteristic odour.
These acids have a characteristic, sour taste. The volatile acids also have a strong repugnant smell, like Parmesan cheese, unwashed gym socks, vomit or even worse!
They form an homologous series.
The first four members are infinitely soluble in water, but as the chain length increases solubility decreases.
 point of 16.6°C, freezes on cold days and is sometimes called 'glacial acetic acid'.
point of 16.6°C, freezes on cold days and is sometimes called 'glacial acetic acid'.
Again the solubility in water of the lower members and their much higher boiling points than the corresponding alkanes is explained by hydrogen bonding.
As in other homologous series as the chain length increases so melting points and boiling points increase.
Chemical properties of the carboxylic acids
They are weak monoprotic acids.
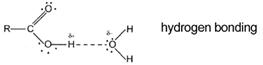
The one transferable proton is in the functional group and attached to an oxygen atom. They ionise incompletely in water (as indicated by the dotted line). Few H+ ions are formed. They are weak acids.
At room temperature, only three to four acetic acid molecules in every 1000 ionise.
They change the colour of indicators.
As weak acids they have excess hydronium ions and will turn blue litmus red. (provided that they are soluble in water).
Cleopatra was Queen of Egypt and one of the earliest women chemists. She demonstrated that acids release carbon dioxide from carbonates when she dissolved her pearls (CaCO3) in vinegar. This was one of the most expensive chemical reactions ever carried out by one person.
They are weak electrolytes.
Partial ionisation gives rise to few ions in solution, and hence they are poor conductors of electricity.
They neutralise alkalis and other bases.
Dilute sodium hydroxide solution is neutralised by organic acids.
CH3COOH(aq) + NaOH(aq) → CH3COONa(aq) + H2O
sodium acetate
They release carbon dioxide from aqueous carbonates and bicarbonates.
The hydrogen ions released by organic acids in water react with carbonates and bicarbonates to form carbon dioxide and water, leaving behind a carboxylate salt.
RCOOH + H2O → RCOO- + H3O+
carboxylate ion
2H3O+ + CO32—→ CO2 + 3H2O
2-
2H3O+ + CO3
→ CO2 + 3H2O
For example, acetic acid will form acetate ions and citric acid will form citrate ions. Sodium acetate has the formula CH3COONa.
The release of carbon dioxide on addition of an aqueous carbonate or bicarbonate solution to an acid is used as a test for the presence of an acid.
The reaction equations can be written like this:
CH3COOH(aq) + NaHCO3 → CH3COONa + H2O + CO2
But it is not the acetic acid molecule that reacts - rather it is the H3O+ or H+ ions.
Esters
Many fruity odours in nature are volatile esters and many artificial flavourings - banana, peach, apricot, pear and rum, to name a few - are esters which are used in cooking, and for ice-cream and milk flavourings.
It may surprise you to know that all animal fats and many oils are esters too and without them, many of our hot foods would lack flavour.
Liquid esters are also good solvents. Ethyl acetate, used in nail polish remover, is an example. Synthetic vinyl leathers contain esters called alkyl phthalates which keep them supple. Such esters are called plasticisers. These esters are responsible for the 'plasticky' smell of plastics.
Esters are formed when an alcohol reacts with an acid.
Esterification
Making an ester from an acid and an alcohol is called esterification. The acid may be either organic or inorganic. The word equation is:
ACID + ALCOHOL → ESTER + WATER
Not a method recommended for
the wine industry where development of esters enhances the wine flavour!
The reaction is slow even when it is heated in the presence of a catalyst. The catalyst used is a trace of a strong mineral acid such as sulfuric acid.
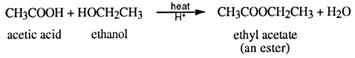
Esterification is also classified as a condensation reaction because water is squeezed out as a condensate when the two reactant functional groups react together. An example is the preparation of ethyl ethanoate (ethyl acetate).
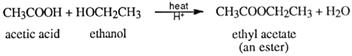
The general equation for the preparation of esters is:
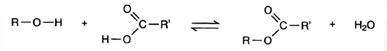
Where R represents an alkyl group or benzene ring, RI is an alkyl group or a benzene ring (or H, if the acid is methanoic).
Naming Esters
Esters are named by a combination of the alcohol and carboxylic acid names from which
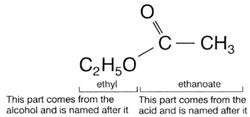
they are made. For example, ethanol and ethanoic acid (acetic acid) form the ester ethyl ethanoate (or ethyl acetate). The ester from propan-1-ol and butanoic acid is called
1-propyl butanoate.
Hydrolysis of esters
Decomposition of esters to form the parent acid and alcohol is called hydrolysis. The simplest hydrolysis (reaction with water) is acid catalysed and is the reverse of esterification.
Esters have two word names (like salts). They are alkyl alkanoates. The alkyl group is named after the parent acid. All esters made from acetic acid (ethanoic acid) have acetate (or ethanoate) as their second name.
Sulfuric acid catalyst
ester + water → acid + alcohol
heat
The reaction works best by boiling the ester with sodium hydroxide solution. The sodium hydroxide neutralises the organic acid formed and the other product, an alcohol is easily separated from the salt by distillation.
e.g. CH3CH2COOCH3 + NaOH(aq) → CH3CH2COONa + CH3OH
methyl propanoate sodium propanoate methanol
If the ester used is an animal fat or vegetable oil the sodium salts formed are soaps. This soap making reaction is widely used on a commercial scale and is discussed in the chapter on biomolecules.
Biomolecules
What are the biomolecules?
This is the collective name given to the many different molecules found in living things. Many biomolecules are relatively small e.g. glucose, C6H12O6, while some are very large complex molecules e.g. DNA, deoxyribonucleic acid. All are essential for life.
Carbohydrates
Carbohydrates occur in all living things and are the major constituents of most plants, making up from 50 to 90% of their dry mass. Starch, cellulose and the sugars are classified as carbohydrates. They are used for food, clothing (linen, cotton, rayon) communication (paper) as well as for fuel and shelter (wood). Historically the term carbohydrate was derived from the molecular formula of glucose, which is C6H12O6 and thought to be the hydrate of carbon C6(H2O)6.
However, there are no water molecules in glucose and this view was quickly abandoned, but the name has stuck.
The most common carbohydrate is also the world's most common organic compound, cellulose. Over half the world's combined carbon is found in this compound. The second most common organic compound is another carbohydrate - starch. Both of these are polymers of glucose.
Photosynthesis makes glucose and oxygen in a complex process where carbon dioxide from the air combines with water, using the sun's energy. This is harvested by green leaf solar collectors which contain the pigment chlorophyll.
One is reminded of the well known fairy story of Hansel & Gretel in the witch's gingerbread house wher e t he c hildr en c ould not di sti ng uish th e foo d fro m th e furniture.
This means that the carbohydrate m olec ules ar e aldehy des or ketones with many (poly) hydroxy (-OH) groups and can be very large molecules (polymers).
The three main components of honey are glucose, fructose and sucrose.

Much of this glucose is chemically linked in chains to form starch for energy storage or the structural material cellulose, which encloses all plant cells and gives plants support and structure.
Carbohydrates are compounds of carbon, hydrogen and oxygen and defined as polyhydroxy aldehydes or ketones, or their polymers. There are three broad classifications:
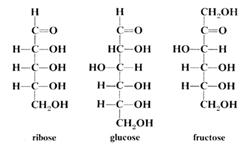
1 Simple sugars or monosaccharides such as glucose or fructose, which cannot be broken down into any smaller sugars. Examples are glucose and fructose - both C6H12O6, and ribose C5H10O5. Most simple sugars contain either 5 or 6 carbon atoms.
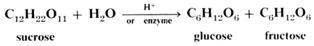
2 Double sugars are also called disaccharides. These are made of two linked simple sugar units and the best known is sucrose or ordinary table sugar. It is
one of the most abundant pure chemicals in the world. Its formula is C12H22O11, not
C12H24O12, as on linking glucose and fructose a molecule of water is eliminated.
The reverse process to reconstitute the simple sugars is called hydrolysis.
3 Complex sugars or polysaccharides have tens, hundreds or thousands of simple sugars chemically linked. The most abundant of these are cellulose and starch and both of these are glucose polymers. Starch itself is actually made of two different glucose polymers, one soluble in cold water and branched; the other unbranched and insoluble in cold water.
Glycogen is another many branched glucose polymer and is used as an energy store for animals. It can contain up to 100,000 glucose units. The formulae of all three can be represented as (C6H10O5)n and, like double sugars, can be hydrolysed into simple sugars. In living organisms this is carried out by specific catalysts called enzymes.
enzyme
(C6H10O5)n + (n-1)H2O → nC6H12O6
starch hydrolysis glucose
Cellulose is indigestible by humans because we lack the right enzymes, which is why we can digest peas and potatoes but not grass, stems and leaves. Starch is present in almost all plants and is an important constituent of many of our foods. Potatoes contain about 20% starch, 77% water, 1% salts and 2% protein. Wheat contains up to 80% starch.
A test for the presence of starch in foods uses iodine. If a dilute iodine solution is added to starch a blue colour forms.
Sugars are most commonly found as 5 or 6 membered ring structures. Some structural formulae are shown below:
Starch granules are insoluble in cold water but in hot water swell and form a paste. This is what happens to starchy foods during cooking.
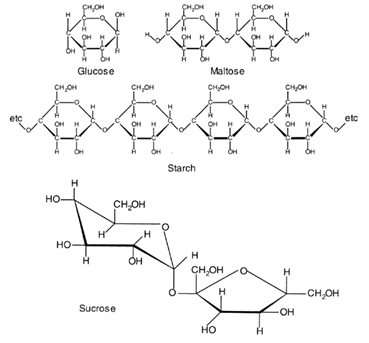 This diagram for sucrose attempts to show the three-dimensional character of one of the carbon rings. The first ring is in the so called ''chair' form; the second ring is planar. The carbon atoms in the ring are not shown.
This diagram for sucrose attempts to show the three-dimensional character of one of the carbon rings. The first ring is in the so called ''chair' form; the second ring is planar. The carbon atoms in the ring are not shown.
Reactions of carbohydrates
Hydrolysis and fermentation are two key processes in bread making and beer-making and in Egypt and Iraq they evolved together. In bread-making one of the products of fermentation is carbon dioxide, which makes the bread rise. In beer and wine making the key product is ethanol. The same fermentation process of the simple sugars, glucose or fructose, provides both.
enzyme
C6H12O6 → 2CH3CH2OH + 2CO2
Fermentation
Fermentation is the action of micro-organisms on compounds to produce different compounds, without using oxygen. The micro-organisms produce enzymes to speed up the fermentation. These micro-organisms, which are yeasts or bacteria, feed on the compounds, and the new compounds are waste products of their fermentation.
• Penicillin is made by fermentation.
• Lactose (milk sugar) can be fermented to form lactic acid which is needed to make cheese.
• The cocoa bean used to make chocolate is fermented to produce the chocolate flavour.
• Soy beans are fermented to make soy sauce.
• Sugars are fermented to give ethanol and carbon dioxide.
Sugars are carbohydrates, they contain carbon, hydrogen and oxygen, and have the general formula, Cx(H2O)y.
• Glucose and fructose have the formula C6H12O6.
• Maltose and sucrose have the formula C12H22O11.
• Starch is (C6H10O5)n.
Notice that each sugar shown has one, two or many C6 units. The value for n in starches can be more than a million.
Sugars are sweet and soluble carbohydrates. Strictly, starch is not a sugar.
Wine almost makes itself. The crushed grape with its coating of yeast ferments on its own.
Fermentation you need to know is:
C6H12O6(aq) → 2CO2(g) + 2C2H5OH(aq)
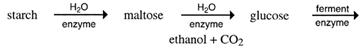
The role of enzymes - The sequence of converting starch into ethanol is as follows:
It is like cracking a large alkane into smaller ones. However, thermal cracking, as done with alkanes, is not possible. Melting sugar in a saucepan shows this. More heat gradually darkens the sugar. It is said to 'caramelise'. The caramel flavour is due to the heat forming new compounds. The C12 sucrose is not split into two C6 sugars.
Enzymes are needed to do this - not only to direct the breakdown of starch, maltose and sucrose into C6 sugars, but also to speed up these reactions. Enzymes are biological catalysts and each different reaction needs a different enzyme.
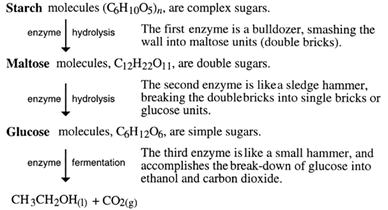
Don't forget the carbon dioxide! It helps bread and yeast buns to rise, and when the moist dough dries out in the baking process, the gas cells remain firm and the bread stays risen. The alcohol evaporates almost completely during the bake. It also provides the bubbles in champagne..
Conditions
It has been said that you can make wine out of any ingredient - even old boots! This is true provided you have some sugar, acid and yeast in a dilute aqueous solution.
Grapes will not grow everywhere and wines from fruits, flowers and roots have been made for centuries. Tinned fruits, dried fruits, dried flowers, dried herbs and even jam, grass, seaweed or grain can be used.
Many fruits contain their own sugar and acid. A yeast nutrient such as diammonium phosphate helps too, and warmth is essential.
The steps in wine making
Grape picking
To pick grapes at the right pH is important.
Too much acid gives an undrinkable, vinegary wine. Too little gives a flat uninteresting taste.
The right amount gives wine a fresh, tart taste.
Grape Crushing
Remove seeds, stems and skins unless the latter are needed for colour (red wine). Grape juice is called 'must'.
Killing wild yeasts with sulfur dioxide
Wild yeasts frequently lead to slow fermentations, as they do not convert all the sugar to alcohol. Odd and/or unpleasant odours and flavours are produced.
Fermentation of the 'must'
For red wine 24 - 26.5°C.
For white wines 10 - 15.5°C (the lower temperatures improve the yield of fruity esters). Fermentation is exothermic and excess heat must be removed using cooling jackets. At about 40°C, yeasts are killed and it is difficult to restart a fermentation.
Allow the 'lees' (sediments) to settle
Racking in stainless steel storage tanks
Many wines are aged in oak barrels. The tannin from the wood adds flavour.
Bottled and corked
Fining agents are added to remove insoluble particles or a centrifuge is used.
Fractional distillation of aqueous alcohol
Both fermentation of simple sugars and hydration of ethene produce very dilute ethanol. Wine is nearly nine-tenths water!
Unless wine is carefully stored it can be subjected to bacterial attack by vinegar- forming bacteria. Concentrated alcoholic solutions are used as antiseptics and preservatives. Nothing can live in them. Fractional distillation separates the more volatile ethanol (T°b 78.3°C/1atm) from the water.
Distillation has been described as the art of turning a liquid into vapour by heat and condensing this vapour by cooling and collecting the condensate (called the distillate), in a separate part of the apparatus.
Did you realise t hat in yeast bakery, fermentation produces ethanol and carbon dioxide? What happens to the alcohol?
The science of wine making is called oenology.
Louis Pasteur discovered that the presence of living yeast was necessary to turn grape juice into wine (1857) and that bacteria cause wine to turn into vinegar (1866).
Wine is made from apples, apricots, beetroot, celery, seaweed, dates figs, pears, peaches, honey, parsnip, quince, rhubarb, rice, honey, potatoes and of course grapes as well as many other fruits and vegetables. Where the sugar content is low, raisins are often added.
The acids in wine are tartaric, acetic, malic, succinic and lactic.
Wine making yeasts contain invertase, an enzyme which converts sucrose into glucose and fructose.
The Latin word mustum means new wine. (Mustard was originally made from the crushed seeds of various plants mixed with grape juice.)
If the temperature changes too much, the complex fermentation reaction produces an undesirable balance of chemicals that will spoil the wine.
Construct a flow chart for wine making using the steps given.
Fermentation, in addition to producing ethanol, produces higher alcohols such as
propan-1-ol and butan-1-ol.
In-situ means inside the reaction place - or on the spot.
Rectify : to purify (especially a spirit or liquor) by repeat ed distillation.
Distillation was known to the Romans and the Greeks over 2000 years ago. A still is used. Vodka, port, sherry, brandy and whisky are all made by distillation and, except for vodka, which is almost tasteless because the distillate is passed through activated charcoal, have distinctive flavours.
Distillation of wine to produce spirits became common from about 1500 onwards. Distillation can also produce some chemical changes as well. Brandy is named from the Dutch 'brandewijn' meaning burnt wine. There are many other chemicals apart from water and ethanol in brandy. Some of the chemicals are produced during the double distillation process, (brandy is distilled twice) and others extracted from the oak used to make the barrels the brandy is stored in.
Both brandy and Scotch whisky are commonly distilled from copper pot stills (a brandy pot still is pictured below) and both brandy and whisky mature in oak casks from two to twenty years.
Some four hundred flavour components have been identified in whisky. These include long chain alcohols, aldehydes, fatty acids and esters from the acetic acids. Some of these esters are extracted from the oak casks or made by slow esterification of compounds in the distillate.
Some of the aldehydes are made by oxidation in-situ of the alcohols in the wine.
Pure water is added to brandy and whisky distillates before maturation. Brandy is 37.0% alcohol by volume.
Proof Spirit
This term is applied to an alcoholic liquor or a mixture of alcohol and water containing
49.28% ethanol by weight or 57.10% by volume.
"Proving the spirit" was a ceremony on British Navy ships when serving out a rum
ration. Gunpowder would just burn if wet with proof spirit, less than this, no ignition.
Rectified spirit, made by fractional distillation of aqueous alcohol solutions, is 96%
ethanol and 4% water by volume.
To obtain absolute alcohol (100% ethanol), it must be distilled with a small amount of benzene. Absolute alcohol in open containers dilutes itself spontaneously. It is hygroscopic, meaning it absorbs water vapour from the air.
Source : http://www.usc.adelaide.edu.au/local/transitionlectures/chemistry/alcohols_carboxylicacids_esters.doc
Web site link: http://www.usc.adelaide.edu.au/
Google key word : Alcohols, Carboxylic Acids and Esters file type : doc
Author : not indicated on the source document of the above text
If you are the author of the text above and you not agree to share your knowledge for teaching, research, scholarship (for fair use as indicated in the United States copyrigh low) please send us an e-mail and we will remove your text quickly.
Alcohols, Carboxylic Acids and Esters
If you want to quickly find the pages about a particular topic as Alcohols, Carboxylic Acids and Esters use the following search engine:
Chemistry
Alcohols, Carboxylic Acids and Esters
Please visit our home page
Larapedia.com Terms of service and privacy page
