Chemical bonding
Chemical bonding
The following texts are the property of their respective authors and we thank them for giving us the opportunity to share for free to students, teachers and users of the Web their texts will used only for illustrative educational and scientific purposes only.
The information of medicine and health contained in the site are of a general nature and purpose which is purely informative and for this reason may not replace in any case, the council of a doctor or a qualified entity legally to the profession.
![]()
Chemical bonding
Chemistry
Introduction
Chemical bonding describes the ideas, models and theories that chemists have about how elements combine to make compounds.
Because there are two types of atoms, metal and non-metal, it is a reasonable assumption that there could be three classes of chemical bonds:
|
• |
those between metal and non-metal atoms |
|
• |
those between non-metal atoms, and |
|
• |
those between metal atoms. |
Is this a useful classification? How can more information be obtained? The answer is, "By experiment". Chemical investigations show that the compounds formed between metal and non-metal atoms have very different properties to those formed from non-metal atoms. And we already know that metals are very different to non-metals.
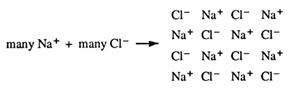
Sodium chloride, NaCl, is a common example of a compound formed from a metal (sodium) and a non-metal (chlorine). It is a crystalline solid, soluble in water, conducts electricity when dissolved in water, does not conduct electricity as a solid, does conduct electricity when molten and has a high melting point.
Other compounds made from metals and non-metals have these general properties. Some examples include potassium chloride, KCl, iron(ll) sulfate, FeSO4, and calcium bromide, CaBr2.
Non-metal compounds show very different properties. Dichloromethane, CH2Cl2, is a compound formed from non-metal atoms. It has a low melting point (it is a liquid at room temperature), it does not conduct electricity and it is not soluble in water. Other non-metal compounds have similar general properties.
While metals have many similar properties. They are hard, shiny, have high melting points and conduct electricity in the solid state.
Is it reasonable, then, to assume that the bonds between metal and non-metal atoms are similar, so giving rise to their similar properties, but these bonds are very different to those between non-metal atoms? And that the bonds holding metal atoms together are completely different again?
Chemists say yes, so the bonding theories about how atoms hold together centre around these three classes:
|
• |
metal: non-metal, |
called the ionic bond; |
|
• |
non-metal: non-metal, |
called the covalent bond; |
|
• |
metal: metal, |
called the metallic bond. |
These experiments indicate that it would be worthwhile finding out more about the bonding classification, according to the kind of atoms that make up the compound. Many chemists have done this and today's bonding theories are centered on these three classes:
|
• |
metal : non-metal |
|
• |
non-metal : non-metal |
|
• |
metal : metal |
Imagination is more important than knowledge!
A. Einstein
Electrons tend to exist in pairs. Four pairs of valence electrons are very stable - the octet rule.
Stable electron configurations
But before we go further we need to look at a group of gaseous elements, collectively called the noble gases. These gases are helium (He), neon (Ne), argon (Ar), krypton (Kr), and xenon (Xe) and are chemically unreactive. Their electron configurations show either a full outer shell of 2 electrons for helium, or 8 electrons for the remainder.
When one examines the electron configuration of metal atoms, these are seen to have fewer electrons in their outer shells than do non-metal atoms. Look at this table; it will confirm this statement.
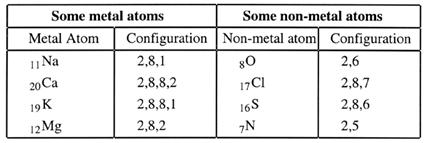
We also know that the noble gases are very stable elements and that their atoms have an outer shell of eight electrons. Can we link their stability to their outer shell electron structures?
 In addition, our observations about matter show that compounds of metals and non- metals are much more stable than their constituent elements. Let us look at the two elements, sodium and chlorine, and their compound, sodium chloride, and compare their physical properties.
In addition, our observations about matter show that compounds of metals and non- metals are much more stable than their constituent elements. Let us look at the two elements, sodium and chlorine, and their compound, sodium chloride, and compare their physical properties.
These summaries are not to be learnt, but simply show the differences in reactivity between the elements and their compounds. The question is now, 'Can the stability of sodium chloride be related to a noble gas electron configuration?' The answer to this is yes. Chemical bonds form when atoms gain noble gas electron configurations.
Before looking further at bonding remember that bonding:
|
• |
is a theory. That atoms are bound together is a fact - why they do so is a theory. |
|
• |
is the word to describe the hold that atoms have over each other. |
|
• |
is electrostatic in nature. |
Chemists say that this hold in all kinds of bonding is due to the attraction that opposite charges have for each other. Static electricity, that is the attraction positive charge has for negative charge, provides the key to how atoms bond to each other.
Electron dot formula
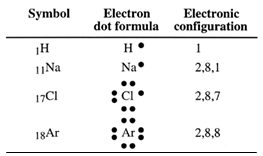 o save time when writing outer electron configurations, chemists use electron dot formulae instead of writing atom structure diagrams. These formulae consist of the symbol for the atom and dots to represent the outer electrons.
o save time when writing outer electron configurations, chemists use electron dot formulae instead of writing atom structure diagrams. These formulae consist of the symbol for the atom and dots to represent the outer electrons.
We can now show how atoms might combine using electron dot formulae.
The ionic bond
Look at the diagram given for the structure of the sodium atom, 11Na. If one outer electron is lost, the electron configuration becomes that of neon, written 2,8.
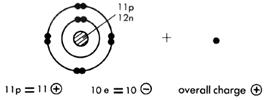
Note that this particle has the nucleus of the sodium atom but the configuration of neon. It has 11 protons but only 10 electrons. It has an overall positive charge and is called a sodium ion. This ion now has a stable outer configuration.
Chemists find it quicker to use electron dot symbols to show this loss of electrons. Electron dots are used only to show valence electrons. The overall charge of one on each sodium ion is shown by +
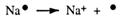
How can electrons be removed from an atom? Heat energy will do this. Heating starts many chemical reactions.
Look at the diagram for the chlorine atom, 17Cl. With seven outer electrons, this atom requires one more electron to get the inert gas structure of eight outer electrons, the stable octet! If heated sodium is placed in chlorine gas, Cl2(g), electrons can be transferred
 The
The
electron dot formulae show this transfer of electrons:
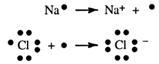
When gaining an extra electron the chlorine atom becomes the chloride ion. This has negative charge. Now we have also learnt that positive attracts negative. All the sodium ions formed when sodium burns in chlorine attract the chloride ions, and all the chloride ions attract the sodium ions. This mutual attraction brings the particles together to form a
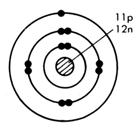
 Sodium atom
Sodium atom
 An ion is a particle with a charge. Particles are charged when the overall number of protons does not equal the total number of electrons. The charge is caused by gaining electrons to make the
An ion is a particle with a charge. Particles are charged when the overall number of protons does not equal the total number of electrons. The charge is caused by gaining electrons to make the
ion negative, or by losing electrons, to make the ion positive.
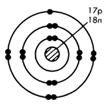
Chlorine atom
 A three dimensional arrangement of ions is called an ionic lattice.
A three dimensional arrangement of ions is called an ionic lattice.
The particles in ionic compounds are ions, which are not paired but exist in an extended 3D-lattice structure. We owe this knowledge to Sir William Lawrence Bragg who was educated in Adelaide.
Bragg studied sodium chloride crystals with X-rays to find the position of the atoms, but found, instead, ions. Few believed him (Bragg was only 22 at the time). However, three years later, he and his father, Sir William Henry Bragg, were jointly awarded the Nobel Prize for Physics.
 n dot structures are also called Lewis Structures. So named to honour G.N. Lewis (1875 -
n dot structures are also called Lewis Structures. So named to honour G.N. Lewis (1875 -
1946) a US scientist and a major contributor to bonding theory.
 Ionic compounds are not made up of molecules.
Ionic compounds are not made up of molecules.
This regular, 3D arrangement of ions is called an ionic latice.
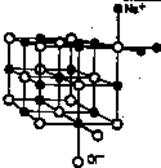
Ionic latice for sodium chloride.
solid is termed an ionic solid because it is made of ions.
 The bonds holding these ions together are termed ionic bonds. The ionic bond is the bond we use to explain how metal and non-metal atoms join together. The ionic bond forms when atoms of metals transfer their outer electrons to atoms of non-metals so that each has a stable noble gas configuration. The force of attraction between the positive and negative ions so formed makes up the ionic bond.
The bonds holding these ions together are termed ionic bonds. The ionic bond is the bond we use to explain how metal and non-metal atoms join together. The ionic bond forms when atoms of metals transfer their outer electrons to atoms of non-metals so that each has a stable noble gas configuration. The force of attraction between the positive and negative ions so formed makes up the ionic bond.
Let us look at another example. When heated, magnesium burns brilliantly in air or oxygen forming a white solid, magnesium oxide, MgO. It is possible to explain the formation of MgO in terms of electron transfer and the formation of ions.
Step 1: Write electron dot symbols for Mg and O.

Step 2: Decide how many electrons must Mg lose and O gain in order to have noble gas configurations? Answer: two each.
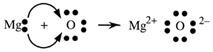
Step 3: Write the electron transfer.
Another example is the formation of magnesium chloride:
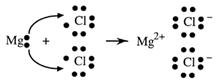
Because each Mg needs to lose two electrons to have a stable outer shell and each Cl can only gain one electron, there must be two Cl atoms for each Mg when reaction occurs. The formula for magnesium chloride, MgCl2, shows this ratio. The compound as a whole is electrically neutral, the two single negative charges (2-) balancing the one double
 positive (2+).
positive (2+).
Properties of ionic compounds
The compounds formed between metal and non-metal atoms are called ionic compounds and have a number of properties in common. These properties are:
1. Ionic compounds are crystalline solids
This property can be explained by realising that the + and - ions attract one another and build up into a giant structure in a regular way. This regularity is responsible for the crystalline shape.
2. Ionic compounds are hard to melt
They have a high melting point. Because of the considerable attraction a positive ion has for a negative ion, a considerable amount of energy is required to
separate the oppositely charged ions. Melting means that the particles separate from each other.

molecules, are called aquated ions
+ -
 effectively reduce the force of attraction between these ions. The structure disintegrates as water takes away the positive and negative ions. Water molecules surround each positive and negative ion, increasing the distance between them and reducing the force of attraction. The water molecules also provide an insulating barrier between charges. This reduces their force of attraction for each other as well. The ionic solid dissolves.
effectively reduce the force of attraction between these ions. The structure disintegrates as water takes away the positive and negative ions. Water molecules surround each positive and negative ion, increasing the distance between them and reducing the force of attraction. The water molecules also provide an insulating barrier between charges. This reduces their force of attraction for each other as well. The ionic solid dissolves.
4. Ionic compounds when molten or when dissolved conduct electricity
The positive and negative ions are able to carry the current in the melt or in the solution. They are free to move.
How to write the formulae of ionic compounds
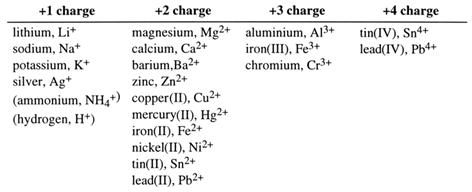
In ionic compounds the number of positive charges is balanced by an equal number of negative charges. The compound formed is neutral. Thus in calcium chloride, the Ca2+ requires two Cl- in order to balance the charges. The formula is CaCl2. This is how the formulae of ionic compounds are worked out. This is easy; what is more difficult is learning the charge on the ions. This table will help you. Note that some atoms can have different charges. Iron, for example, can form Fe2+ or Fe3+. Numbers are used to show which iron ion is required, when two or more are possible:
Fe2+ is iron(II). Fe3+ is iron(III).
Common ions of metals

Common ions of non-metals
Polyatomic ions
These are the ions with many atoms. Some ionic compounds contain ions made up of more than one type of atom. There are many important negatively charged ions of this sort. Those with a (•) are the ones you should remember now (others will come into the course later).
e.g. Na (aq) Cl (aq)
 Metal ions have positive charges
Metal ions have positive charges
 l ions have negative charges
l ions have negative charges

 The competent chemist is able to write formulae with ease. Remember, the formula indicates what kind and how many atoms or ions are present, e.g.
The competent chemist is able to write formulae with ease. Remember, the formula indicates what kind and how many atoms or ions are present, e.g.
2 Cl- represents two separate chloride ions.
Cl2 represents a molecule of chlorine containing two bonded chlorine atoms.
K2S indicates that the combining ratio of potassium ions to sulfide ions is 2:1. (The subscript 2 refers to the potassium.)
Na2CO3 indicates a combining ratio of two sodium ions to each
2-
carbonate ion ( carbonate is CO3
, so that's one of them, not 3! )
 y.
y.

atoms molecules
CuCl2 indicates a combining ratio of one copper to two chloride ions.

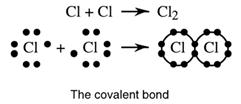
The name given to the chemical bond formed by non-metal atoms sharing electrons is covalent. Co means to share and refers to the sharing of electrons, and valent comes from a Latin word meaning strength; strength by sharing. The covalent bond forms when non-metal atoms share electrons. Each atom, as a result of this sharing, obtains a stable outer shell of electrons. It is the force of attraction between the positive nucleus and the shared negatively charged electrons which joins the atoms together.
Role of electrons in bonding
Gaseous elements, for example oxygen, hydrogen, chlorine and nitrogen, exist as two atoms joined together. They are diatomic molecules. The noble gases do not join together. They are monatomic. These things can be understood by referring to electron structures again.
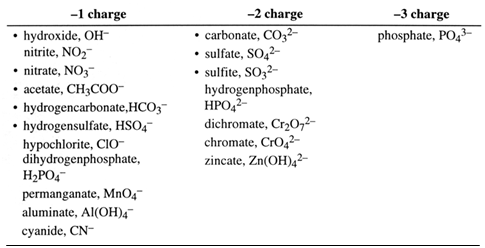
When two hydrogen atoms come close together they join to form a hydrogen molecule. This is a fact. There can be no argument about hydrogen molecules containing two atoms of hydrogen. Diagrams for the structure of the hydrogen atoms can be written like this:
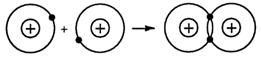
The hydrogen molecule is held together by the electrostatic forces of attraction between one nucleus, a shared pair of electrons, and the other nucleus.
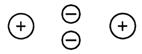
This shared pair of electrons spends most of its time, on average, in a volume of space between the two positively charged nuclei.

The electron clouds of the two atoms overlap,
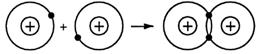
and so the diatomic molecule looks as drawn below.
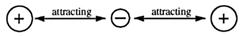
When the electron shells overlap there is a force of attraction between the positive nucleus of the atoms and the negative electrons.
This is our theory. Chemists may yet come up with a better idea to explain how atoms hold together. Note that our theory uses:
• the attraction between opposite charges:
• the attaining of a stable outer configuration and sharing of electrons. Each atom
now has a share of the other's electrons.
There is an extension of this theory to explain why the electrons hold together. It is assumed that the electrons are spinning. The physicists tell us that spinning electrons have associated with them a magnetic field and the nature of this magnetic field is dependent on the way the electrons spin. If the electrons are spinning in opposite directions, then they attract each other. Electrons with the same spins repel each other.
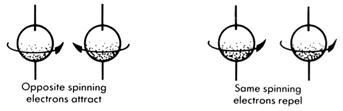
 .
.
 e circle outlines are used as a check that each atom has its full outer shell.
e circle outlines are used as a check that each atom has its full outer shell.
Naming covalent compounds
If only two elements are present, prefixes 'mon-', 'di-', 'tri-', 'tetra-' etc. are used on the name of the second element to indicate how many atoms of it are present in the formula.
CO carbon monoxide CO2 carbon dioxide SO3 sulfur trioxide
N2O4 dinitrogen tetroxide
Chlorine atoms have seven outer electrons. By sharing one electron from each atom, a stable configuration of 8 can be obtained.
A group of eight outer electrons is called an octet and the tendency for atoms to group so as to get eight electrons each is called the octet rule. Again the force bonding the two atoms together is the attraction between the positive nuclei and the shared electron pair.
Chemists use a dash ( - ) to show a covalent bond. This represents one shared electron pair.
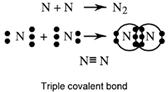
Double and triple covalent bonds are collectively called multiple bonds.
Now you know why the formula of water is H2O!
These compounds formed by the covalent bonding of non-metal atoms are called molecules.
Cl _ Cl
Covalent bond
More than one pair of electrons can be shared. Oxygen has six outer electrons and so needs two electrons in order to complete its octet. This is achieved in the oxygen molecule by sharing two pairs of electrons.
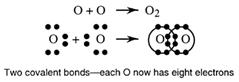
Two covalent bonds are termed a double bond.
O = O
A triple bond has three shared electron pairs. N2 is an example. Each N atom has five outer electrons and so needs three extra electrons to complete its octet.

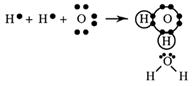
Water, H2O(l)
Oxygen has six and hydrogen has one outer electron. By sharing, each can obtain a stable outer shell - an octet and a duet.
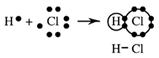
Hydrogen chloride, HCl(g)
Carbon dioxide, CO2(g)
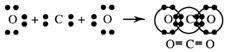
Ammonia, NH3(g)
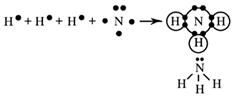
Methane, CH4(g)
Polar covalent bonds
You will learn later in this chapter why chemists prefer to draw the molecules using lines the way shown here. A clue: it is related to the shape of the molecule!
The definition for an ionic bond is: The force of attraction between two oppositely charged ions.
A covalent bond is defined as the force of attraction of a shared electron pair for the positive nuclei of the two participating atoms - the force of attraction of positive for negative again.
The sharing of electrons by atoms is not always equal. In fact it is only equal when atoms of the same non-metal elements form covalent bonds.
A covalent bond where there is an unequal sharing of the shared electrons is called a polar covalent bond or just a polar bond. This occurs between two different non-metal atoms as these have different electronegativities. Electronegativity is a term chemists use to compare the attraction of an atom for a shared pair of electrons when it is bonded to another atom.
The more electronegative element has a greater share of the shared pair and this gives it a partial negative charge. The other element, having a smaller share has a partial positive charge. A chemist shows this using the Greek delta, δ which means a small amount - less than one, so partial positive is written δ+ and partial negative is written δ - on the formula of the compound itself as shown for hydrogen chloride below.
δ+ δ-
H ─ Cl polar covalent bond
The delta positive and delta negative are equal and opposite. Molecules have no overall charge.
Electronegativity
Electronegativity compares the attraction of an atom for a shared pair of electrons when it is bonded to another atom. Now since one electron of the shared pair comes from each atom anyway, electronegativity can also be regarded as the force of attraction for an extra valence electron. Elements with a high electronegativity have a strong force of attraction for an extra electron. Not surprisingly, the most reactive non-metal element fluorine, has the highest electronegativity. Even metals can be assigned electronegativity values but metals are elements which lose electrons in a chemical change forming the corresponding cations, so metals, particularly the active ones in groups 1 and 2 have low electronegativities. These elements are sometimes referred to as being electropositive. The Pauling electronegativity scale is used, with fluorine, oxygen, nitrogen and chlorine being the most electronegative and rubidium, caesium and francium being the least.
Why is it so?There are two factors and one is size.
The effect of size on electronegativity
Down a group the size increases progressively as an extra layer of electrons (the outer shell) increases the atomic radius and the atomic volume. These are now the new valence electrons and further from the positive nucleus and so more weakly held. The force of attraction between positive and negative decreases with distance - quite dramatically in fact. The mathematical relationship is the force (F) of attraction is inversely proportional to the distance (d) squared, written:
F α 1
d2
Across a period from groups 1 to 7 the size decreases and so across a period the electronegativity increases.Why does the atomic radius across a period decrease? The period number gives the shell number. Across a period there is no change in the number of shells but there is an increase left to right in the number of valence electrons with a corresponding number of extra protons in the nucleus. This brings in the second factor, core charge, sometimes called effective core charge and sometimes residual core charge.
The effect of core charge on electronegativity
In order to simplify an atom, the nucleus and inner shell electrons can be considered the core of the atom and the outer shell electrons the valence electrons. Hence sodium with
11 protons and 10 inner shell electrons has a core charge of +1 attracting its one outer electron. All elements in this period have 10 inner shell electrons but progressively one more proton and an extra valence electron, so magnesium's core charge is 12 - 10 = +2, aluminium's core charge is 13 - 10 = +3 and chlorine in group 7 has a core charge of 17 -
10 = +7. Core charge is easy to work out because numerically it equals the group number.
The bigger the core charge of an atom, the stronger will be the attraction. There is a direct proportion between size of charge and attraction. Two positives will attract twice as much as one.
F the force of attraction is proportional to the charges +ve and -ve.
F α charge 1 x charge 2
Combining these two we get F α charge 1 x charge 2 which is Coulomb's Law
d2
As the core charge of an atom of an element increases, its attraction for valence electrons increases. Thus the atomic radius shrinks left to right across the periodic table from groups
1 to 7.
 In period 3, sodium has the biggest atomic radius and chlorine the least. Chlorine has the highest electronegativity in period 3. The diagram below shows the trend in electronegativity up a group and across the periodic table with the corresponding values on the Pauling scale. Remember the trends, not the actual values.
In period 3, sodium has the biggest atomic radius and chlorine the least. Chlorine has the highest electronegativity in period 3. The diagram below shows the trend in electronegativity up a group and across the periodic table with the corresponding values on the Pauling scale. Remember the trends, not the actual values.
H
2.1
Li Be B C N O F
1.0 1.5 2.0 2.5 3.0 3.5 4.0
Na Cl
0.9 3.0
Electronegativity difference
Bond polarity depends upon the electronegativity difference of the two participating atoms. H ─ F with an electronegativity difference of 4.0 - 2.1 = 2.9 (see table above) is more polar than the remaining hydrogen halides, and of these three, HI has the least polar covalent bond. (The electronegativity difference between H and I in H ─ I is 2.5 - 2.1 =
0.4), but you do not have to do calculations. Just be aware of the trends in
electronegativity. Which bond is more polar, H ─ S or H ─ O?
The shapes of molecules
 can be measured. The actual shapes of many molecules have been determined by experiment in the laboratory and there is a very close correlation between the theory (bonding) and the fact (the experimental evidence). Because of this correlation, chemists are reasonably happy with their bonding theory.
can be measured. The actual shapes of many molecules have been determined by experiment in the laboratory and there is a very close correlation between the theory (bonding) and the fact (the experimental evidence). Because of this correlation, chemists are reasonably happy with their bonding theory.
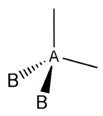
In predicting the shapes of molecules, the following assumptions are made:
• The central atom is spherical in shape.
• The central atom in a molecule will usually have four pairs of electrons (an octet)
for its final electronic configuration.
• These electron pairs will arrange themselves on the surface of the sphere so as to give the maximum distance between pairs. By gaining maximum distance there is the minimum repulsion between pairs. We assume each pair acts as a negative charge.
Tetrahedral shape
Let us use these assumptions to predict the shape of molecules.
Methane, CH4
Step 1
Draw the electron dot formula for CH4.
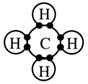
There are four electron pairs around the central carbon atom.
This theory is known as V S E P R
- Valence Shell Electron Pair
Repulsion theory.
Tetra means four and hedron means a solid figure. A tetrahedron is a four-sided figure in which each face is an equilateral triangle. A tetrahedral shape is based on the tetrahedron.
Step 2
Arrange these four pairs of electrons for maximum distance, that is, minimum repulsion. To get the idea of maximum distance, your hands will help.
Place the forefinger and thumb of both hands opposite each other so that the distance between the forefingers and the thumbs is the same as the distance between the hands. This makes a square.
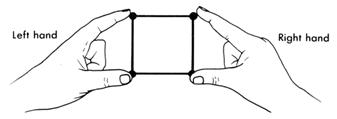
This is not the maximum distance between the four pairs. Turn the right had at right angles to the left.
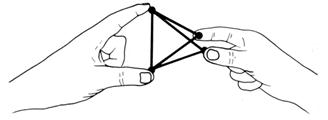
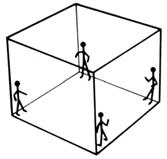
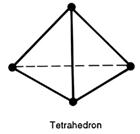
When four strangers travel in a lift, they stand as far away as possible from each other.
Four flies in a lift can get further away from each other.
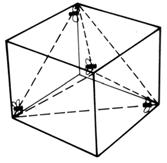
If the lift is a cube, the shape made by joining the shown positions is a regular tetrahedron
and gives the maximum possible distance between four equally spaced points.
If the centre of a tetrahedron is joined to its four corners, the angle between any two corners and the centre is 109.5° - the tetrahedral angle.
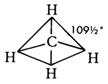
The theory and experiment agree. Thus the shape of a methane (CH4) molecule is drawn as
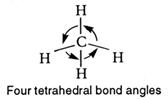
and the angle HCH is termed the bond angle.
There are just five different molecular shapes that you need to know. These are linear, bent, trigonal plane, trigonal pyramid and tetrahedral. Whenever only two atoms are present the shape must be linear.
The Valence Bond Electron Pair Repulsion theory or VSEPR defines a region of electron density as being a single, double or triple bond or a non-bonding pair of electrons. These regions of electron density around a central atom repel each other to give the lowest energy and hence the most stable structure. The bonded atoms then describe the shape which is dictated by all regions of electron density - also called charge centres.
Regions of Electron density (charge centres) |
Electron region shape |
Molecular Shape |
Example |
2 |
Linear Bond angles 180o |
Linear |
|
3 |
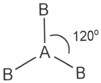
Trigonal planar Bond angle 120o |
Trigonal planar |
|
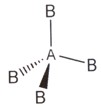
4 |
Tetrahedral Bond angle 109.5o |
Tetrahedral |
|
Trigonal pyramid |
|
||
Bent or V - shaped |
|
W e do the best we can to represent a three-dimensional shape in two dimensions. The diagram
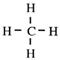
is not acceptable to chemists for the shape of the methane molecule, CH4, as it suggests a square with angles of 90°. Each bond angle in t he methane molecule is 109.5° so this is not
Non-bonding pairs of electrons are m or e r epuls iv e t han bonded electron pairs.
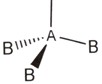
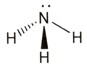
Ammonia, NH3
Step 1
Draw the electron dot formula NH3
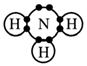
Step 2
Arrange these electron pairs for maximum distance.
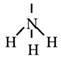
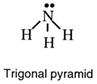
This gives a tetrahedral arrangement of electron pairs, but only three of these pairs are bonding pairs. The fourth pair has no atom joined to it and is called a non-bonding or lone pair. The structure resulting is called a trigonal pyramid and is usually drawn as:
Water, H2O
Step 1
Draw the electron dot formula for H2O.
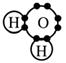
Step 2
Arrange these electron pairs for maximum distance. This is, again, a tetrahedral arrangement.
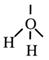
Water has two non-bonding pairs of electrons and the resultant structure is called
V-shaped or bent and is drawn as such.
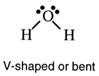
When the measured angle between atoms in a molecule is less than the predicted angle, the explanation is found in the extra repulsion given by non-bonding pairs of electrons. In water, the angle HOH is less that 109.5° because the two non-bonding pairs push the bonding pairs closer together. The bond angle in water is close to 105°.
Polar covalent molecules
Two factors determine whether or not a molecule is polar. One is that polar bonds must be present but this on its own does not guarantee that the molecule is polar. Look at the structure of CO2 and CCl4. Both are non-polar because the polar bonds are arranged symetrically to give zero net direction of change. Put another way, the centres of positive and negative charge coincide.
The second factor is 3D structure. The shapes of CO2 and CCl4 have cancelled out individual bond polarities.
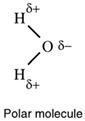
Small polar molecules have a δ+ end and a δ- end or, 'the centres of positive and negative charge do not coincide' - they are separated because of the bond polarities and because of their molecular shapes.
Both polar and non-polar molecules have no net charge so the two δ+ on the hydrogens of
the water molecule shown are equal and opposite to the one δ- on the oxygen.
Identifying Polar Molecules
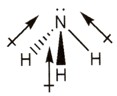
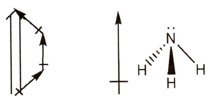
Consider ammonia, NH3. Its shape has been given previously as a trigonal pyramid.
|
Decide if the bonds are polar and draw these on your diagram as shown. |
|
Each bond in ammonia links nitrogen to hydrogen.
There is a substantial difference in electronegativity between nitrogen and hydrogen but not enough to make this an ionic bond. Since nitrogen is more electronegative than hydrogen the bond dipoles are shown with the arrows on the nitrogen end. |
|
On adding the individual dipoles together, the resulting molecular dipole can be drawn.
This shows that the partial negative end is where the nitrogen is and the partial positive end is where the hydrogens are. NH3 is polar overall! |
Working with molecular models makes identification of polar molecules easier to visualize. With practice you can do this at a glance. Both physical and chemical properties are affected by the polarity of molecules as you will see.
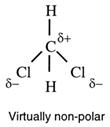
The C _ H bond is almost non- polar. T heref or e, c ompounds containing only C and H atoms (called hydrocarbons) are almost non-polar.
The equal and opposite forces cancel each other out. The result is a non-polar molecule.
In carbon tetrachloride there is no net direction of charge. The result is a non-polar molecule.
These charges almost cancel out. The result: almost a non-polar molecule.
When there is an uneven spread of electrons making a covalent bond, the bond is called a polar covalent bond. When there is an uneven spread of electrons over a molecule giving an unequal spread of charge over the molecule, the molecule is said to be polar. Water is an example of a polar molecule. Like bond polarity, molecular polarity is also shown using δ- and δ+.
Consequently, polar molecules arise when there is a net direction of charge over the molecule: polar bonds arranged in such a way as to give a net direction of charge. However, there are some instances when the polar bonds are arranged symmetrically so as to give zero net direction of charge; this is a non-polar molecule. For example, carbon dioxide,
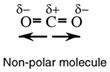
and carbon tetrachloride,
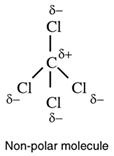
Note that non-polar bonds can never give rise to polar molecules. Some molecules have very low polarity - so low as to be regarded as non-polar,
Properties of covalent compounds
Compounds with covalent bonds have a number of properties because of the type of bonding present.
1 Covalent compounds are usually low melting-point solids, or liquids or gases.
The reason for this is that the chemical bonds are between the atoms inside the molecule, not between the molecules. Water is a liquid at room temperature. Methane is a gas.
In the solid, the molecules are arranged in regular crystalline patterns held together by relatively weak forces of attraction. Ice crystals are an example of a molecular crystal- water molecules make up the pattern.
The very weak forces between molecules are collectively known as secondary bonds. There are different types of secondary bonds, known as dispersion forces (van der Waal's forces), dipole-dipole interactions and hydrogen bonds. The strongest of these forces is no stronger than about one tenth that of a covalent bond.
A few covalently bonded substances (e.g. carbon, silicon, and silicon dioxide) have very high melting points. They form continuous covalent lattices.
Covalent bonds are formed between atoms of non-metals. They are very strong bonds, binding atoms together into m olec ules . T hes e m olecules usually have little attraction for e a ch o th e r, ma ki n g co va l e n t compounds easy to melt.
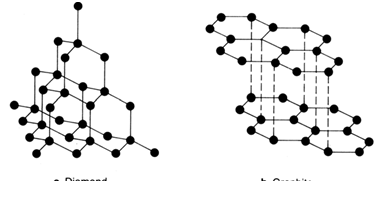
a Diamond b Graphite
 2 Covalent
2 Covalent
compounds do not conduct electricity.
They are neutral particles and the bonding electron pairs are localised between the bonded atoms. Because of this no conduction is possible. There are no ions.
3 Most covalent compounds tend not to dissolve in water.
However, there are some important exceptions (e.g. HCl(g)). We will deal with these later.
4 Covalent compounds tend to dissolve in covalent solvents other than water.
Covalent solvents include alcohol, C2H5OH, methylene chloride, CH2Cl2, and chloroform, CHCl3.
5 Covalent compounds are usually very stable.
Covalent compounds are usually very stable because the covalent bond is a strong bond. They often need strong heating in order to get them to react, and even then
their reactions may be slow. When a covalent molecular substance changes state, the covalent bonds are not broken; only the weak secondary bonds between molecules break.
Covalent lattices
When the molecules of a solid are arranged in a fixed geometrical pattern, the substance is said to be crystalline. Just as in patterned wallpaper or floor tiles we can see a unit of pattern which keeps repeating itself in two dimensions over and over again, we can examine the three-dimensional structure of a crystal and see that it has a basic arrangement of molecules that keeps repeating too.
More allotropes of carbon have been made. These are called the fullerenes, the first being a C60 molecule. They are named after an American architect, Buckminster Fuller. He designed 'geodesic domes' and his structures, on a macroscale, are similar, on a microscale, to the structure of the fullerenes. Another name could be
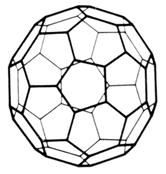
Special tiles with covalent bonds t hroughout cover t he space shuttle. These tiles are tough and have a very high melting point. They protect the shuttle from the t r emendous heat generat ed between the shuttle and the atmosphere on re-entry.
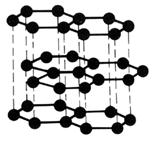
The layer structure of graphite, showing the planar hexagonal units.
The underlying geometrical pattern of any crystalline substance is called the lattice of the substance. Sometimes the lattice is an arrangement of atoms, (e.g. C) sometimes an arrangement of molecules, (e.g. CO2 in Dry Ice).
Diamond, the hardest naturally occurring substance known, often occurs as single crystals of great beauty and regularity. Diamond is pure carbon, and the arrangement of atoms in the lattice has influenced the shape of the crystal. Carbon forms another lattice structure called graphite, and both diamond and graphite are examples of a non-metal element with a very high melting point.
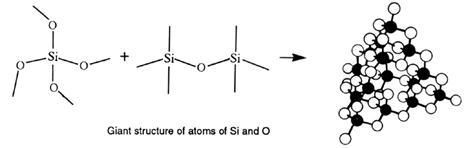
There are also some compounds of non-metals, which are extremely hard to melt. Silicon dioxide, SiO2 (sand), is an example. It has a melting point of 1700°C. Because non-metal atoms are present, the bonding must be covalent. Why then do they have such high melting points?
The answer is that they form covalent bonds throughout the structure. All atoms are joined by covalent bonds. As the covalent bond is very strong, considerable energy is required to overcome the forces holding the solid together. The bonds in SiO2 are continuous. Each Si is joined to four O atoms and each O is joined to two Si atoms - there are no molecules of SiO2.
The formula, SiO2, is an empirical formula - the simplest whole number ratio. It can be understood if one realises that each silicon has a half share of four oxygen atoms (4 x 1/2 =
2!)
In diamond, each C is joined to four other C atoms and each is tetrahedrally surrounded by four other C:
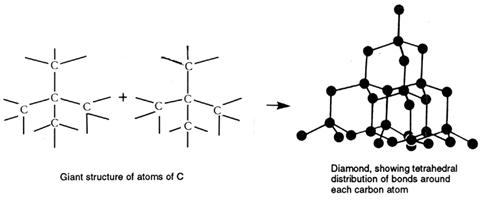
These structures, consisting of atoms joined by covalent bonds in three dimensions, are termed giant atomic crystals, or continuous covalent lattices (or networks). Giant, because they contain millions of atoms even in the smallest crystal, atomic because atoms are present within the crystal, and crystals because there is a regular arrangement of particles within the structure.
Graphite has a layered structure with each carbon atom bonded to three other carbons in hexagonal rings within each layer. The extra (fourth) valence electron from each carbon then is available to form a strong, delocalised bond between the layers. This arrangement
accounts for the high melting point, electrical conductivity (electrons between the layers are free to move) and lubricant (layers slide over each other) properties of graphite.
Naming binary compounds of the non-metals
You have learned that when two non-metallic elements combine to form a binary (or
"two-element") compound, the result is a covalent compound.
Hydrogen forms binary compounds with all of the non-metals. Except in a few cases the H atom is written first in the formula and is named first. The other non-metal is named as if it were a negative ion. For example:
COMPOUND |
NAME |
HCl H2S |
hydrogen chloride hydrogen sulfide |
Many of the binary compounds of non-metals were discovered years ago and have names so common that they continue to be used. Some that you need to know include:
COMPOUND |
COMMON NAME |
H2O H2O2 NH3 NO |
water hydrogen peroxide ammonia nitric oxide |
Most of the binary, non-metal compounds you will see have at least one element from Group 6 or 7 of the Periodic Table. This element is always listed second in the formula and is named second. The number of such elements in the molecule is designated with a prefix such as "di-, tri-, tetra-", and so on. For example:
COMPOUND |
NAME |
NO2 N2O4 PCl5
NF3 |
nitrogen dioxide dinitrogen tetroxide phosphorous pentachloride nitrogen trifluoride |
The metal bond
We rely very much on the bonds between metal atoms. We expect a piece of iron to be strong, and use iron to make the products that have to stand up to stress and shock. When you next see an aeroplane ask, 'What is holding it all together?' This is a different question to, 'Why does it fly?' The answer lies in the bonds between the atoms of aluminium. These bonds we call the metal bonds. They are the bonds between particles in metals.
Before moving on to the metal bond, it is necessary to summarise some of the properties we associate with metals:
Metal atoms bond through the sharing of each other's electrons. The number of electrons available
for sharing determines the properties of metals. Each sodium atom has one electron available, a magnesium atom has two, and an aluminium atom has three. Because of this they have different properties.
|
• |
Metals are good conductors of electricity and heat. |
|
• |
Most metals have a high density. |
|
• |
Most metals have high melting point. |
|
• |
Most metals are strong - they can be bent without breaking and they can be |
|
|
flattened without shattering, and they can be drawn into wires. |
The ideas we develop about the structure and bonding in metallic substances must be able to support these general properties of metals.
The metal bond forms because metal atoms lose control of their outer electrons to other metal atoms and these in turn lose control of the electrons to other atoms and so on. These outer electrons are able to move freely from one atom to another. Because the metal atom has lost its outer electrons it is now a positive ion. These positive ions are
attracted to the wandering electrons. This is an electrostatic attraction and a strong one. The wandering electrons are shared loosely between their nearest atoms. This, too, provides attraction between the particles.
Perhaps the best way of attempting to picture the metal bond is to think of a metal as positive ions embedded in a moving 'sea of valence electrons', or the electrons as providing the 'glue' to stop the positive ions from flying apart. The metal atoms are arranged in a regular, close-packed lattice.
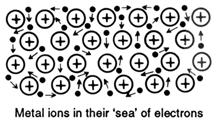
Electrical conductivity can be likened to water flowing through a pipe - put water in at one end and instantly water flows out the other.
This is a regular lattice structure
Metals are good conductors of electricity and heat because the electrons are free to move in the metal. Electricity pushes electrons into one end of a wire and out they come at the other end. The moving electrons still provide the attraction between the positive and negative needed to hold the metal together. Switch off the electricity and the electrons in the wire continue to wander freely but are not pushed about.
If a piece of metal is heated at one end, the atoms begin to vibrate more quickly. These vibrations are transferred throughout the metal. In this way heat is conducted throughout a metal.
Their 'cementing' electrons pack the ions tightly and strongly together. Because no space is wasted in this packing, metals are dense and considerable energy must be supplied in order to overcome the bonding. Most metals are hard to melt.
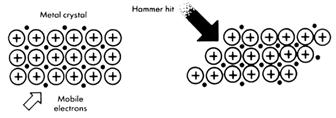
When a metal is bent or struck with a hammer, the positive ions will move around, but they are still held by their electrons. As the electrons move with the ions, metals do not shatter when hit, unlike ionic compounds. Ionic compounds shatter because the positive and negative ions are forced apart when stressed. Unlike the small electron, negative ions cannot move freely.
Ionic crystals
These shatter when hit because ions with the same charge repel each other.

Metal crystals
Yes, metals are crystals, because the positive ions are packed together into a regular pattern. But, when hit, unlike charges are still next to each other and the solid does not shatter. The structure is unchanged-the forces of attraction are still present.
Using the periodic table
The periodic table is a chemical filing cabinet where each element has a number, 1 to 118, which is its atomic number. The development and full significance of the periodic table is best left until later. However, you will observe:
1 The elements are listed in increasing order of atomic number.
2 There are three blocks - the one on the left hand side contains metals only. The rectangular place in the middle also contains metals only.
3 The block on the right hand side is the only one with non-metals. These are in the top right hand corner.
4 The left hand and right hand blocks have eight vertical rows labelled in Roman numbers I to VIII. These vertical rows are called groups and the Roman numbers are group numbers. You can use these group numbers to find the combining powers of main group elements when they form salts.
Consider groups I to IV. The group number is the same as the valency. This means all metals in Group I have a valency of 1; all metals in group II have a valency of 2 and so on. Consider groups V to VIII. The valency can be given as eight minus the group number.
Group number |
Valency |
V VI VII VIII |
8 - 5 = 3 8 - 6 = 2 8 - 7 = 1 8 - 8 = 0 |
Note that for groups V to VIII, these valencies apply in salts. Difficulties arise in using them for covalent compounds because they apply in some cases only.
Metals in the middle do not have a group number. They are simply called transition metals and include the important metals iron, copper, zinc, nickel, silver and gold. Transition metals have variable valencies.
Source : http://www.usc.adelaide.edu.au/local/transitionlectures/chemistry/bonding.doc
Web site link: http://www.usc.adelaide.edu.au/
Google key word : Chemical bonding file type : doc
Author : not indicated on the source document of the above text
If you are the author of the text above and you not agree to share your knowledge for teaching, research, scholarship (for fair use as indicated in the United States copyrigh low) please send us an e-mail and we will remove your text quickly.
Chemical bonding
If you want to quickly find the pages about a particular topic as Chemical bonding use the following search engine:
Chemistry
Chemical bonding
Please visit our home page
Larapedia.com Terms of service and privacy page