Gases & the kinetic molecular theory
Gases & the kinetic molecular theory
The following texts are the property of their respective authors and we thank them for giving us the opportunity to share for free to students, teachers and users of the Web their texts will used only for illustrative educational and scientific purposes only.
The information of medicine and health contained in the site are of a general nature and purpose which is purely informative and for this reason may not replace in any case, the council of a doctor or a qualified entity legally to the profession.
![]()
Gases & the kinetic molecular theory
Chemistry
Gases & the Kinetic Molecular Theory
Properties of Gases
- Matter exists in three states: solids, liquids, and gases.
- A vapor is a gas that has formed either by the evaporation of a liquid or the sublimation of a solid.
- The density of gases is significantly less than liquids and solids – this indicates that the gas molecules are far apart relative to their size and their interactions are weak
- Gases are easily compressed and fill their container
- The interactions of gases would be minimal b/c of the molecules are so far apart if were not for their rapid motion.
- Properties of Gases:
- Gases can be compressed into smaller volumes; their densities can be increased by increasing pressure
- Gases exert pressure on their surroundings; therefore, pressure must be exerted to be confined
- Gases expand w/out limits; so gases can uniformly and completely occupy the volume of any container
- Gases diffuse into one another; meaning that gases placed in the same container mix completely and cannot separate on standing
- The amounts and properties of gases are describe in terms of temperature, pressure, volume occupied and number of molecules
Basics
- Pressure = force/area
- What is a barometer?
- What is a manometer?
- Know the units of pressure: torr, mm Hg, and atm
- Be able to convert b/t all 3 units of pressure: 1 atm = 760 mm Hg at 0oC = 760 torr
- The SI unit of pressure is the pascal (Pa)
- 1 atm = 101.325 kPa
Boyle’s Law
- Know Boyle’s Law: expresses the relationship between volume and pressure
P1V1 = P2V2 (constant n, T) Remember: n = moles
Charles’s Law
- Know Charles’s Law: expresses the relationship between volume and temperature (in Kelvin)
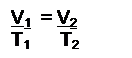
(constant n, P)
STP
- Standard Temperature and Pressure (STP) = 0oC (273 K) and 1 atm
Combined Gas Law
- Know the Combined Gas Law
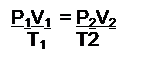
(at constant n)
Avogadro’s Law
- Know Avogadro’s Law
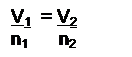
(at constant T,P)
- Avogadro’s Law led to the Standard Molar Volume: 1 mole of a gas = 22.4 L at STP
Ideal Gas Law
- Know the Ideal Gas Law
- PV = nRT
- R is the Universal Gas Constant
- R = 0.0821 L atm/ mol K
- R = 8.314J/mol K
- You decide which R to use based on the units
Additional Information
- Be able to do solve for molecular weights of gases using the Ideal Gas Law
- By solving for n you can get moles; then using the provided mass solve for the molecular weight
Dalton’s Law
- Know Dalton’s Law of Partial Pressure – the total pressure exerted by a mixture of gases is the sum of the partial pressures of the each gas.
Ptotal = P1 + P2 + P3 + …
- For gases collected over water you must take into consideration that the gas is saturated with water vapor. (Patm is the atmospheric pressure)
Patm = Pgas + Pwater
- So if you are trying to solve for the pressure of the gas:
Pgas = Patm - Pwater
Gas Stoiciometry
- Be able to complete stoichiometric calculations for reactions with gases.
- Remember that for gases 22.4 L = 1 mol at STP
- If the conditions are not at STP you must use the Ideal Gas Law to solve for moles and then do your stoichiometry
KMT
- Know the Kinetic Molecular Theory
- #1. Gas is composed of particles- usually molecules or atoms
- #2. Particles in a gas move rapidly in constant random motion
- #3. Collisions are perfectly elastic, no energy is lost or gained
- #4. Between collisions, the molecules exert no attractive or repulsive forces on each other
Diffusion/Effusion
- Know what diffusion is.
- Know what effusion is.
- How does molecular size affect effusion/diffusion
- From a group of substances be able to determine which would have greatest rate of effusion/diffusion
- Know Graham’s Law
Real Gases
- Know how real gases deviate from ideal gases
- Non-ideal gas behavior is greatest when the gas is at high pressure and/or low temp. ( near liquefaction)
Source : http://teachers.greenville.k12.sc.us/sites/jhushen/AP%20Chemistry%20Resources/Chapter%205%20Gases/AP%20Chemistry%20Chapter5%20Study%20Guide.doc
Web site link: http://teachers.greenville.k12.sc.us
Google key word : Gases & the kinetic molecular theory file type : doc
Author : not indicated on the source document of the above text
If you are the author of the text above and you not agree to share your knowledge for teaching, research, scholarship (for fair use as indicated in the United States copyrigh low) please send us an e-mail and we will remove your text quickly.
Gases & the kinetic molecular theory
If you want to quickly find the pages about a particular topic as Gases & the kinetic molecular theory use the following search engine:
Chemistry
Gases & the kinetic molecular theory
Please visit our home page
Larapedia.com Terms of service and privacy page
