Basic chemistry notes
Basic chemistry notes
The following texts are the property of their respective authors and we thank them for giving us the opportunity to share for free to students, teachers and users of the Web their texts will used only for illustrative educational and scientific purposes only.
The information of medicine and health contained in the site are of a general nature and purpose which is purely informative and for this reason may not replace in any case, the council of a doctor or a qualified entity legally to the profession.
![]()
Basic chemistry notes
Chemistry
Basic Chemistry Review
Element: a substance that cannot be broken down into simpler chemical substances.
Atom: smallest particle of an element that still has the properties of that element.
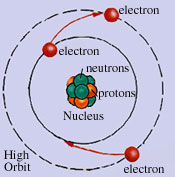
Nucleus: center of an atom. Atomic nuclei are composed of 2 subatomic particles:
Protons: Positively charged
Large in size
The number of protons in the nucleus determines the identity of an
element. This is the atomic number of the element.
Neutrons: Neutral charge
Same size as protons
Neutrons add mass to the nucleus. The number of protons + the
number of neutrons in the nucleus is an element’s atomic mass or
mass number.
Electrons are negatively charged particles that orbit the nucleus in an electron cloud. The electron cloud is divided energy levels and within each energy level there are sublevels. Each energy level can hold a specific number of electrons:
1st energy level holds up to 2 electrons (s sublevel holds both electrons)
2nd energy level holds up to 8 electrons (s sublevel holds 2 and the p sublevel holds 6)
3rd energy level holds up to 18 electrons (s holds 2, p holds 6 and the d sublevel holds 10)
4th energy level holds up to 32 electrons (s holds 2, p holds 6, d holds 10 and the f sublevel holds 14)
Electrons are very small. They are so small the mass of an individual electron cannot be measured.
In an atom, the number of protons=the number of electrons, atoms have no electric charge
Isotopes: atoms of the same element that have a differing number of neutrons in their nuclei. The
Different number of neutrons gives isotopes different atomic masses.
Ex. Carbon-12 6 protons Carbon-14 6 protons
6 neutrons 8 neutrons

Chemical bonding and Chemical compounds
Compound: substance composed of two or more elements that have been combined chemically.
The properties of a compound are different than the properties of the elements that make it up.
Ex. 2H2 + O2 à 2H2O
H2 and O2 are both highly flammable, but water is used to put fires out.
Chemical bonds hold compounds together. There are two main types of chemical bonds:
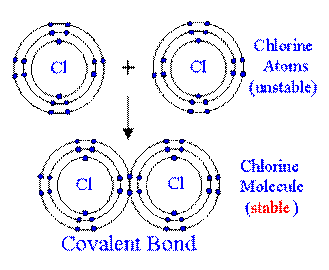
Covalent bonds: form when atoms share electrons to form a compound. Covalent bonds form
molecules.
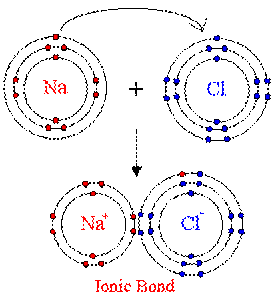
Ionic bonds: form when electrons are transferred from one atom to another. Ions are atoms
have gained or lost electrons, so they have an electrical charge. Atoms that gain
electrons have a negative charge, atoms that lose electrons have a positive charge.
Ionic bonds do not form molecules.
Chemical equations
Chemical equations must obey the Law of Conservation of Matter, so the number of atoms available before a reaction must equal the number of atoms available after the reaction. This is called a balanced equation.
Ex. 2H2 + O2 à 2H2O
4 atoms H2 4 atoms H2
2 atoms O2 2 atoms O2
6 atoms 6 atoms
Every chemical reaction has 2 parts: the reactants (that join together chemically) and the products
(what is formed in the reaction.)
Ex. 2H2 + O2 à 2H2O
reactants product
Solutions and Mixtures
Mixture: combination of substances where the individual components retain their own properties.
Ex. Sugar mixed with sand.
Mixtures can be separated by physical processes.
Solution: a mixture where one substance (the solute) is dissolved in another (the solvent).
Water is the universal solvent.
Acids and Bases
Acid: a substance that released H+ ions when mixed with water.
Acids have a pH of 0 – 6.
Ex. HCl à H+ + Cl-
Base: a substance that releases OH- ions when mixed with water.
Bases have a pH of 8 – 14.
Ex. NaOH à Na+ + OH-
A pH of 7 is neutral. It is neither acid nor base.
The pH scale measures the concentration of H+ ions in solution. The greater the concentration of hydrogen in the solution, the lower the pH. As the concentration of hydrogen in the solution decreases,
The pH gets higher.
The pH scale shows an exponential relationship. Each number on the pH scale represents a factor of 10. What does this mean?
An acid with a pH of 0 is 10 times stronger than an acid with a pH of 1 and it is 1 000 000
times stronger than an acid with a pH of 6.
Acids and bases have the ability to neutralize each other. When an acid and a base of equal strength are mixed, the result is a neutral solution.
Ex. HCl + NaOH à NaCl + H2O
Every neutralization reaction will result in an ionic compound that is dissolved in water and the pH will always be 7.
Indicators
Indicators are chemicals that change color in the presence of other chemicals. There are many indicators that tell whether a solution is acid or base, but these are some of the most common:
Litmus paper: blue litmus turns red in an acid
red litmus turns blue in a base
Bromthymol blue: blue in a base and yellow in an acid
Phenolthelein: colorless in an acid and pink in a base
Properties of Water
Water is a polar molecule. This means that it bonds covalently, but electrons are not shared equally between the hydrogen atoms and the oxygen atom.
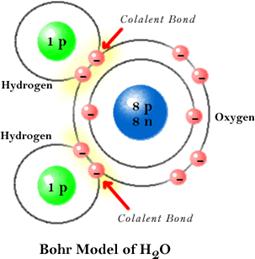
Since more electrons can be found orbiting the oxygen portion of the molecule, that end has a negative charge. Since fewer electrons can be found around the hydrogen atoms that end has a positive charge.
Because of the difference in charge the molecule acts like a magnet. The positive hydrogen end of the molecule attracts negative ions and compounds and the negative end attracts positive ions and compounds.
Because of this attraction, ionic compounds (ex. Salt) and polar compounds (ex. Sugar) dissolve in water.
Water molecules also attract other water molecules. This attraction forms a weak bond called a hydrogen bond. Hydrogen bonds hold many important molecules together (ex. Proteins).
The polarity of water is responsible for many of the unique properties of water.
Adhesion is the tendency of water to stick to the walls of its container.
Responsible for capillary action
Cohesion is the tendency for water molecules to stick to each other.
Responsible for surface tension
Because of cohesive forces, it is difficult to move water molecules apart. For this reason, water is very slow to gain heat. Once the molecules have been moved apart, though they are difficult to move back together. For this reason, water is slow to lose heat, once it has been warmed.
Cohesive forces are also the reason that water expands when it freezes. The hydrogen bonds between water molecules are frozen in place, which makes the molecules less mobile, causing them to remain farther apart.
Movement of molecules
Molecules of all substances are constantly in motion and are constantly trying to distribute themselves evenly. They do this spontaneously without the use of energy.
Diffusion is the movement of molecules from areas of higher concentration to areas of lower concentration. (Follows the concentration gradient.)
Factors affecting diffusion:
Concentration
Most important factor
Steep concentration gradient = fast diffusion
Temperature
Higher temperature = fast diffusion
Pressure
Higher pressure = fast diffusion
Osmosis is the diffusion of water across a cell membrane
Facilitated diffusion is the diffusion of large molecules using carrier molecules.
Active transport IS NOT a type of diffusion.
It moves molecules from areas of low concentration to higher concentration (against the
concentration gradient.)
It uses energy.
Source : http://teacherweb.com/LA/MandevilleHighSchool/Decker/Basic-Chemistry-Notes.doc
Web site link: http://teacherweb.com/LA/MandevilleHighSchool/Decker
Google key word : Basic chemistry notes file type : doc
Author : not indicated on the source document of the above text
If you are the author of the text above and you not agree to share your knowledge for teaching, research, scholarship (for fair use as indicated in the United States copyrigh low) please send us an e-mail and we will remove your text quickly.
Basic chemistry notes
If you want to quickly find the pages about a particular topic as Basic chemistry notes use the following search engine:
Chemistry
Basic chemistry notes
Please visit our home page
Larapedia.com Terms of service and privacy page
