Chemistry gases
Chemistry gases
The following texts are the property of their respective authors and we thank them for giving us the opportunity to share for free to students, teachers and users of the Web their texts will used only for illustrative educational and scientific purposes only.
The information of medicine and health contained in the site are of a general nature and purpose which is purely informative and for this reason may not replace in any case, the council of a doctor or a qualified entity legally to the profession.
![]()
Chemistry gases
Chemistry
- Molecular compounds are converted to gases much more easily than ionic compounds upon heating
- Molecular compounds usually boil at much lower temperatures than ionic compounds
- The stronger intermolecular forces, the less likely a compound can exist as a gas at ordinary temperatures
- Physical characteristics of gases:
- assume the volume and shape of their containers
- are the most compressible of the states of matter
- will mix evenly and completely when confined to the same
container
- have much lower densities than liquids and solids
Pressure of a Gas:
Gases exert pressure on any surface with which they come in contact, because gas molecules are constantly in motion.
SI unit: Pascal (Pa), defined as one Newton per square meter
Pressure = force/area 1 Pa = 1N/m2
Atmospheric Pressure is the pressure exerted by the Earth’s atmosphere. The actual value of atmospheric pressure depends on location, temperature and weather conditions. The barometer measures atmospheric pressure. Standard atmospheric pressure (1 atm) is equal to the pressure that supports a column of mercury exactly 760 mm (or 76 cm) high at 00C at sea level. In other words, the standard atmosphere equals a pressure of 760 mmHg, where mmHg represents the pressure exerted by a column of mercury 1 mm high. The mmHg unit is also called the torr.
1 torr = 1 mmHg 1 atm = 760 mmHg = 760 torr
1 atm = 101 325 Pa 1 atm = 1.01325 x 102 kPa
A manometer is a device used to measure the pressure of gases other than the atmosphere. Two types:
- closed tube – below atmospheric pressure
- open tube – equal to or greater than atmospheric pressure
Examples showing conversions from mmHg to atm and kPa:
THE GAS LAWS:
The Pressure-Volume Relationship: Boyle’s Law
As the pressure (P) is increased at a constant temperature (T), the volume (V) occupied by a given amount of gas decreases. There is an inverse relationship between volume and pressure of a gas at constant temperature. As the pressure increases, the volume decreases. Conversely, if the applied pressure is decreased, the volume the gas occupies increases.
Boyle’s Law: the pressure of a fixed amount of gas at a constant temp is
inversely proportional to the volume of the gas
P is proportional to 1/V
P = k1 x 1/V (where k1 is the proportionality constant)
PV = k1
The product of the pressure and volume of a gas at constant temperature and amount of gas is a constant.
Although the individual values of pressure and volume can vary greatly for a given sample of gas, as long as the temperature is held constant, and the amount of gas does not change, P x V is always equal to the same constant. For a given sample of gas under two different sets of conditions at constant temperature,
P1V1 = k1 = P2V2 or P1V1 = P2V2
where V1 and V2 are the volumes at pressures P1 and P2 respectively.
The Temp-Volume Relationship: Charles’s and Gay-Lussac’s Law
At constant pressure, the volume of a gas sample expands when heated and contracts when cooled. At any given pressure, the plot of volume versus temperature yields a straight line. By extending the line to zero volume, we find the intercept on the temperature axis to be – 273.150C. At any other pressure, we get the same zero volume temperature intercept. We identify -273.150C as absolute zero, theoretically the lowest attainable temperature. An absolute temperature scale was set up, now called the Kelvin temp scale, with absolute zero as the starting point. On the Kelvin scale, 1 Kelvin is equal in magnitude to 10C. The only difference between the two scales is that the zero position is shifted.
The dependence of the volume of a gas on temperature is given by:
V is proportional to T
V = k2T or V/T = k2 where k2 is the proportionality constant
Charles Law: the volume of a fixed amount of gas maintained at constant
pressure is directly proportional to the absolute temperature of
the gas
We can compare two sets of volume-temperature conditions for a given sample of gas at constant pressure.
V1/T1 = k2 = V2/T2 or V1/T1 = V2/T2
where V1 and V2 are the volumes of the gas at temperatures T1 and T2 respectively.
Another form of Charles’s law shows that at constant amount of gas and volume, the pressure of a gas is proportional to temperature:
P is proportional to T P = k3T or P/T = k3
P1/T1 = k3 = P2/T2 or P1/T1 = P2/T2
where P1 and P2 are the pressures of a gas at temperatures T1 and T2 respectively.
The Volume-Amount Relationship: Avogadro’s Law
At the same temperature and pressure, equal volumes of different gases contain the same number of molecules (or atoms, if the gas is monatomic). The volume of any gas must be proportional to the number of moles of molecules present. That is:
V is proportional to n V = k4n
where n represents the number of moles and k4 is the proportionality constant
Avogadro’s Law: at constant pressure and temperature, the volume of a gas
is directly proportional to the number of moles of the gas
present
The Ideal Gas Equation
Summary of the Gas Laws:
Boyle’s Law: V 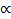 1/P (at constant n and T)
1/P (at constant n and T)
Charles’s Law: V 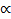 T (at constant n and P)
T (at constant n and P)
Avogadro’s Law: V 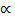 n (at constant P and T)
n (at constant P and T)
We can combine all three expressions to form a single master equation for the behaviour of gases:
V 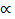 nT/P = R nt/P or PV = nRT (The Ideal Gas Equation)
nT/P = R nt/P or PV = nRT (The Ideal Gas Equation)
where R is the proportionality constant, called the gas constant.
The Ideal gas equation describes the relationship among the four variables P, V, T and n. An ideal gas is a hypothetical gas whose pressure-volume-temperature behaviour can be completely accounted for by the ideal gas equation.
R = 0.0821 L ·atm/K · mol
STP = standard temperature and pressure (00C and 1 atm)
The ideal gas equation is useful for problems that do not involve changes in P, V, and n for a gas sample. At times, we need to deal with changes in these variables. When conditions change, we use a modified form of the ideal gas equation that takes into account initial and final conditions.
P1V1/T1 = P2V2/T2
before change = after change
n1 is usually equal to n2 because the amount of gas normally does not change
Examples:
- Calculate the pressure (in atm) exerted by 1.82 moles of the gas, SF6, in a steel vessel of volume 5.43 L at 69.50C.
- Calculate the volume (in L) occupied by 7.40 g of NH3 at STP.
- An inflated helium balloon with a volume of 0.55 L at sea level (1.0 atm) is allowed to rise to a height of 6.5 km, where the pressure is about 0.40 atm. Assuming the temperature remains constant, what is the final volume of the balloon?
- A certain light bulb containing Ar at 1.20 atm and 180C is heated to 850C at constant volume. Calculate its final pressure (in atm).
- A small bubble rises from the bottom of a lake, where T = 80C and P = 6.4 atm, to the water’s surface, where T= 250C and P = 1.0 atm. Calculate the final volume (in mL) of the bubble if its initial volume was 2.1 mL.
Source : http://www.horton.ednet.ns.ca/staff/coldwell/Chemistry%20(4%20folders%20-%20Anita)/AP%20CHEMISTRY/Gases,%20Liquids,%20&%20Solids/AP%20CHEMISTRY%20-%20Gases.doc
Web site link: http://www.horton.ednet.ns.ca
Google key word : Chemistry gases file type : doc
Author : not indicated on the source document of the above text
If you are the author of the text above and you not agree to share your knowledge for teaching, research, scholarship (for fair use as indicated in the United States copyrigh low) please send us an e-mail and we will remove your text quickly.
Chemistry gases
If you want to quickly find the pages about a particular topic as Chemistry gases use the following search engine:
Chemistry
Chemistry gases
Please visit our home page
Larapedia.com Terms of service and privacy page
