Composition of matter
Composition of matter
The following texts are the property of their respective authors and we thank them for giving us the opportunity to share for free to students, teachers and users of the Web their texts will used only for illustrative educational and scientific purposes only.
The information of medicine and health contained in the site are of a general nature and purpose which is purely informative and for this reason may not replace in any case, the council of a doctor or a qualified entity legally to the profession.
![]()
Composition of matter
Chemistry
Chemistry Notes 
Composition of Matter
Ø Everything in the universe is made of matter
Ø Matter takes up space & has mass
Ø Mass is a measure of the amount of matter in the substance
Ø Mass & weight are NOT the same
Ø Weight is a measure of the pull of gravity on an object
Question: Is the mass of an object the same on the moon as it is on the Earth? Is its weight the same? (Hint: Gravitational pull on the moon is 1/6 of that on the Earth.)
Ø Matter exists in 4 states – solid, liquid, gas, & plasma

Ø Solids have both a definite volume & definite shape (rock)
Ø Liquids have a definite volume but no definite shape; they can be poured (water)
Ø Gases do not have a definite volume or definite shape, but they take the volume & shape of their container
Ø Plasmas have no definite volume, no definite shape, and only exist at extremely high temperatures such as the sun
Ø Chemical Changes in matter are essential to all life processes
Ø Biologists study chemistry because all living things are made of the same kinds of matter that make up nonliving things
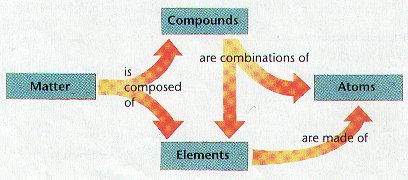
Elements
Ø Elements are pure substances which cannot be chemically broken down into simpler kinds of matter
Ø More than 100 elements have been identified, but only about 30 are important in living things
Ø All of the Elements are arranged on a chart known as the Periodic Table
Ø Periodic charts tell the atomic number, atomic mass, & chemical symbol for every element
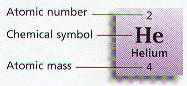
Ø Four elements, Carbon – C, Hydrogen – H, Oxygen – O, and Nitrogen – N make up almost 90% of the mass of living things
Ø Every element has a different chemical symbol composed of one to two letters
Ø Chemical symbols usually come from the first letter or letters of an element like C for Carbon and Cl for Chlorine
Ø Some chemical symbols come form their Latin or Greek name such as Na for Sodium (natrium) or K for Potassium (Kalium)
Ø Elements in the same horizontal period on the periodic table have the same number of energy levels (e.g. H & He in period 1 have only a K energy level)

All Period 2 elements have 2 energy levels
(K & L)
Ø Elements in the same vertical Family on the periodic table have the same number of electrons in their outermost energy level & react similar (e.g. Family IV, the Carbon family all have 4 electrons in their outermost energy level)
Atoms
Ø Atoms are the simplest part of an element that keeps all of the element’s properties
Ø Atoms are too small to be seen so scientists have developed models that show their structure & properties
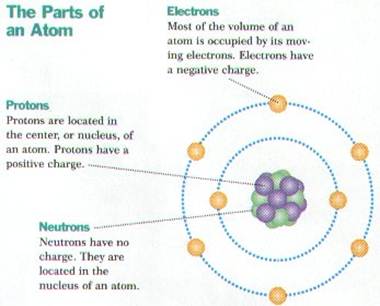
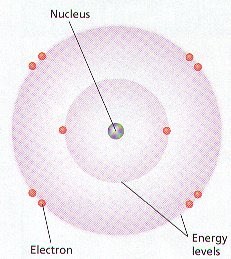
Ø Atoms consist of 3 kinds of subatomic particles – protons & neutrons in the center or nucleus, and electrons spinning in energy levels around the center
Ø The nucleus is the center of an atom where most of the mass is concentrated
Ø Protons are positively charged ( p+ ), have a mass of 1 amu (atomic mass unit) , are found in the nucleus, and determine the atomic number of the element
Example: Carbon has 6 protons so its atomic number is 6
Ø Neutrons are neutral or have no electrical charge (n), have a mass of 1 amu, are found in the nucleus, and when added to the number of protons, determine the atomic mass of the element
Example: Sodium has 11 protons and 12 neutrons so its atomic mass is 11+12=23 amu
Ø Electrons (e-) are negatively charged, high energy particles with little mass that spin around the nucleus in energy levels
Ø Seven energy levels (K, L, M, N, O, P, & Q) exist around the nucleus and each holds a certain number of electrons
Ø The K energy level is closest to the nucleus & only holds 2 electrons, while the L – Q energy levels can hold 8 electrons
Ø Electrons in outer energy level are traveling faster & contain more energy than electrons in inner levels
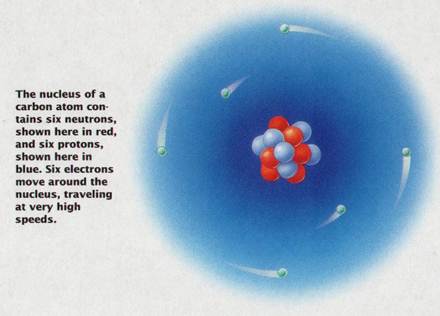
Ø The number of protons (positive charges) and electrons (negative charges in an atom are equal so the net electrical charge on a atom is zero making it electrically neutral
Ø Stable or non-reactive atoms have an outer energy level that is filled with electrons
Compounds
Ø Most elements do not exist by themselves; Most elements combine with other elements
Ø Compounds are made of atoms of two or more elements chemically combined
Ø Chemical Formulas represent a compound & show the kind & number of atoms of each element (e.g. H2O has 2 hydrogen & 1 oxygen)
Ø Compounds have different physical & chemical properties than the atoms that compose them (e.g. hydrogen & oxygen are gases but H2O is a liquid)
Ø The number & arrangement of electrons in an atom determines if it will combine to form compounds
Ø Chemical reactions occur whenever unstable atoms (outer energy level not filled) combine to form more stable compounds
Ø Chemical bonds form between atoms during chemical reactions
Types of Chemical Bonds
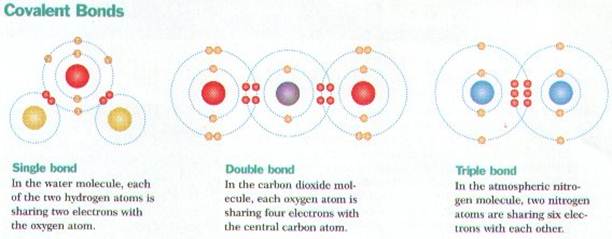
Ø Covalent bonds form between atoms whenever they share 1 or more pairs of electrons (e.g. H2O)

Ø Molecules form from covalent bonding & are the simplest part of a compound (e.g. NaCl, H2O, O2)
Ø Ionic bonding occurs between a positively & negatively charged atom or ion
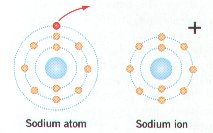
Ø Positively charged ions have more electrons (-) than protons (+); negatively charged ions have more protons than electrons
Ø Table salt (NaCl) forms when the 1 outer electron of Na is transferred to the outer energy level of chlorine that has 7 electrons (e-)
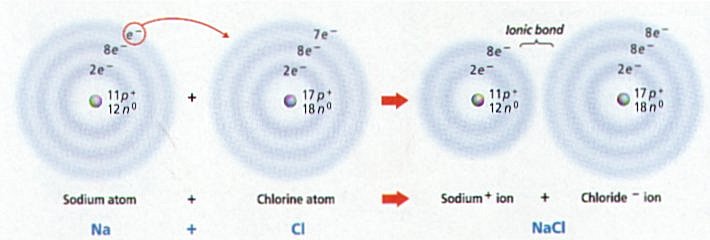
Ø Sodium (Na) with 1 less e- becomes positively charged, while Chlorine (Cl) with 1 more e- becomes negatively charged; the + and – charges attract & form the ionic bond holding NaCl together
Ø Other types of chemical bonding include hydrogen bonding
Energy
Ø Energy is the ability to do work
Ø Energy occurs in several forms & may be converted from one form to another
Ø Sunlight is the ultimate energy for all life on earth
Ø Forms of energy include chemical, electrical, mechanical, thermal, light, & sound
Ø Free energy is the energy available for work (e.g. cells have energy to carry out cell processes)
Ø Cells convert the chemical energy stored in food into other types of energy such as thermal & mechanical
Ø Energy is used to change matter form one state into another (e.g. liquid into a gas)
Chemical Reactions
Ø Living things undergo thousands of chemical reactions
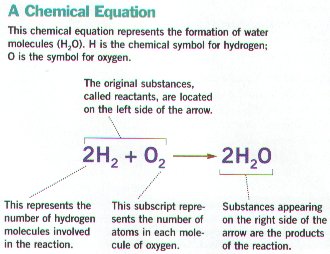
Ø Chemical equations represent chemical reactions
Ø CO2 + H20-----goes to-----H2CO3 (carbonic acid) is a sample Chemical Reaction in living things
Ø Reactants are on the left side of the equation, while products are on the right side
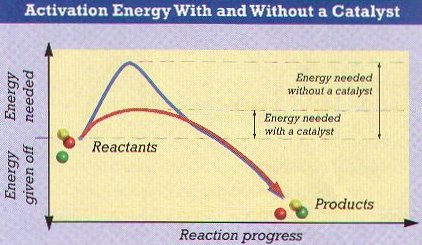
Activation energy is required to start many reactions
Ø Chemical bonds are broken, atoms rearranged, and new bonds form in chemical reaction
Ø Plants use sunlight to produce sugars such as C6H12O6 glucose; the chemical energy from the sun is stored in the chemical bonds of glucose
Ø Organisms eat plants, break down the sugars, and release energy along with CO2 & H2O
Ø Exergonic reactions involve a net release of energy; while endergonic reactions involve a net absorption of energy
Ø Energy must be added to the reactants for most chemical reactions to occur; called activation energy
Ø Enzymes are chemical substances in living things that act as catalysts & reduce the amount of activation energy needed
Ø Organisms contain thousands of different enzymes
Ø Most enzymes end with –ase (e.g. lipase is the enzyme that acts on lipids)

Reduction-Oxidation (Redox) reactions
Ø Reactions in which e- are transferred between atoms is a redox or reduction-oxidation reaction (e.g. formation of table salt NaCl)
Ø In oxidation reactions, a reactant loses 1 or more e- & becomes positively (+) charged (e.g. Sodium atom becomes a Na+ ion)
Ø In a reduction reaction, a reactant gains 1 or more e- and becomes negatively (-) charged (e.g. Chlorine atom becomes a Cl- ion)
Ø REDOX reactions always occur together; the electron(s) from the oxidation reaction are then accepted by another substance in the reduction reaction
Solutions
Ø A large percentage of the mass of organisms is water & many of the chemical reactions of life occur in water
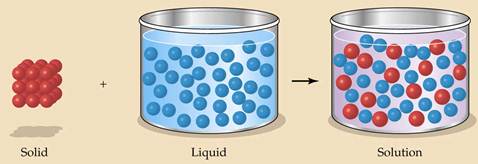
Ø A solution is a uniform mixture of one substance in anther
Ø Solutions may be mixtures of solids, liquids, or gases
Ø The solute is the substance uniformly dissolved in the solution & may be ions, molecules, or atoms
Ø The solvent is the substance in which the solute is dissolved
Ø Water is known as the universal solvent 
Ø Dissolving one substance in another does not alter their chemical properties
Ø The concentration of a solution is a measure of the amount of solute dissolved in a given volume of solvent
Ø Increasing the amount of solute increases the solution’s concentration
Ø Aqueous solutions are solutions in which water is the solvent; these are important in living things (e.g. blood, cytoplasm of cell...)
Acids and Bases
Ø The degree of acidity or alkalinity (basic) is important in organisms
Ø The force of attraction between molecules is so strong that the oxygen atom of one molecule can actually remove the hydrogen from other water molecules; called Dissociation
Ø H20-----GOES TO----- H+ + OH-
Ø OH- called hydroxide ion; H+ called hydrogen ion
Ø Free H+ ion can react with another water molecule to form H3O+ (hydronium ion)
Ø Acidity or alkalinity is a measure of the relative amount of H+ and OH- ions dissolved in a solution
Ø Neutral solutions have an equal number of H+ and OH- ions
Ø Acids have more H3O+ ions than OH- ions; taste sour; and can be corrosive
Ø Bases contain more OH- ions than H3O+ ions; taste bitter; & feel slippery
Examples of Common Acids
|
Examples of Common Bases
|
PH Scale
Ø Compares the relative concentration of H3O+ ions and OH- ions
Ø Scale ranges from 0 to 14; 0-3 is very acidic; 7 is neutral; 11-14 is very basic or alkaline

Ø Litmus paper, phenolphthalein, pH paper, & other indicators that change color can be used to measure pH

Buffers
Ø Control of pH is important to organisms
Ø Enzymes function only within a narrow pH range; usually neutral
Ø Buffers neutral acids or bases in organisms to help control pH
Source : http://gneissscience.wikispaces.com/file/view/Chemistry+Notes+Biology.doc
Web site link: http://gneissscience.wikispaces.com
Google key word : Composition of matter file type : doc
Author : not indicated on the source document of the above text
If you are the author of the text above and you not agree to share your knowledge for teaching, research, scholarship (for fair use as indicated in the United States copyrigh low) please send us an e-mail and we will remove your text quickly.
Composition of matter
If you want to quickly find the pages about a particular topic as Composition of matter use the following search engine:
Chemistry
Composition of matter
Please visit our home page
Larapedia.com Terms of service and privacy page
