Thermodynamics laws explained with examples for kids
Thermodynamics laws explained with examples for kids
The following texts are the property of their respective authors and we thank them for giving us the opportunity to share for free to students, teachers and users of the Web their texts will used only for illustrative educational and scientific purposes only.
All the information in our site are for educational uses.
The information of medicine and health contained in the site are of a general nature and purpose which is purely informative and for this reason may not replace in any case, the council of a doctor or a qualified entity legally to the profession.
Thermodynamics laws explained with examples for kids
Thermodynamics
The universe is a big conglomerate of constantly changing forms of energy. The energy itself does not change, only the form in which the energy takes place. As stated by Albert Einstein, energy cannot be created or destroyed. Thermodynamics chooses to ignore all forms of energy except for heat and the conservation thereof and how it is transformed into mechanical energy. Heat energy can be found in many different places—lava, atomic bonds, friction, chemicals, lightning, solar radiation—and can be harvested in many extraordinary ways. Geothermal machines turn the energy from the Earth into electricity for consumers, nuclear weapons utilize the energy in the nucleus of Uranium and Plutonium through nuclear fission, and solar panels absorb ultraviolet rays and transform the energy into electrical currents usable to the average consumer appliance. Thermodynamics covers all aspects of heat and its transformation to mechanical energy, but does not cover any of the other forms energy may take.
Thermodynamics is confined by two laws. First, heat cannot be created or destroyed, otherwise known as the conservation of heat. Heat being the pure form of energy, neither can be created nor destroyed. Heat can be transformed into to other forms of energy, but it cannot simply disappear. Second, the flow of energy must go from the object of higher temperature to the object of lower energy unless there is the presence of an agent. As will be discussed later on, all natural transfers of heat will obey this law and transfer not from the object of extreme cold to the object of extreme heat but the other way around. The word law, but its very nature, means that whatever is in question has been tried and tested by many different scientists and has yielded the same results every time. Heat cannot be destroyed, nor can it flow from low to high.
Heat itself is simply pure energy; to harvest and use this energy is the goal of the human race. Without heat energy humans could not have advanced to even the Bronze Age in technology. Heat is the energy that warms the Earth and keeps every living organism on Earth alive. Without this energy the trees would die and the planet would become a big glacier floating in space. This energy can be measured in calories or Joules. A calorie is exactly 4.184 Joules; the Joule being the SI unit for heat and energy. Though the Joule is the SI unit, curiously, the calorie is more commonly used to measure this kind of energy. A Calorie or kilocalorie could be used, but the measurements would be very small unless the object being tested was a nuclear warhead.
The opposite of heat is cold. In the back of Conceptual Physics by Paul G. Hewitt, the definition of cold is nowhere to be found. This may seem puzzling unless one examines this dilemma from the chemistry perspective. In chemistry, cold is defined simply as the absence of heat. Perhaps, like a black hole, heat is forced into the void in an attempt to reach equilibrium. A void cannot, however, flow into an object, as stated in the second law of thermodynamics. Also, a definition of something that does not exist will not be found in the glossary of a text book. Unlike heat, cold is not a physical force, it is simply a lack of force. The process of heat transfer must flow from hot to cold because there is only one object actually flowing. All objects on Earth strive to reach equilibrium of heat energy: hot objects are always cooling and cold objects are always heating. Though many of these processes may take longer than the life of the Earth itself, they press on unhindered.
The first practical application of cold and refrigeration was in 1834 by Jacob Perkins. Perkins used ether in a constant evaporation and compression cycle to wick the heat away from the immediate area. Astoundingly almost 100 years earlier, in 1748, William Cullen had already discovered refrigeration. Unfortunately Cullen did not put his invention to any practical use and the world was forced to rely on ice brought from the mountains for refrigeration at home. Refrigeration itself is simply removing the heat energy from objects. This could be the removal of heat from meat, water, fruits, vegetables, milk, or anything really. In modern refrigerators, a chemical called Freon is used. Freon has a low vaporization point and when circulated, it takes the energy from the immediate area and puts it into a change of phase from liquid to gas. Remembering that the heat of vaporization is 540 J per Kg, a small amount of Freon could cool an entire room. Freon wasn’t always the chemical used though; from 1800 to 1929, toxic gases such as ammonia, methyl chloride, and sulfur dioxide were used as refrigerants. Not surprisingly in the 1920’s there was a few gas leaks in some of the refrigerators and many people died as a result. Shortly thereafter, three American companies decided to find a chemical that could be safely placed in the home without the threat of toxic materials next to the food; Freon was the result. One part of the refrigeration cycle not discussed yet is that of the compression cycle. Obviously once the gas has been vaporized it must be returned to a liquid state before it can be vaporized again. In modern refrigerators electricity is used to compress the gas back into its previous state. During this compression cycle, energy is released, and logic says this energy should be able to power the next compression cycle, but technology to do so has not yet been invented.
Speaking of refrigeration and the removal of heat energy, one cannot continue without discussing cryogenics. Contrary to common belief, cryogenics is not he preserving of a human body. Popular movies such as Avatar and Riddick portrait cryogenics as a way to stop the body from aging during space travel. In the future this may become a possibility, but right now it’s as realistic as walking on the surface of the sun. Instead, this area of heat removal is used in the preservation of food, human tissue, blood, and other objects. Cryogenics, by definition, covers all things of the temperature negative 150 degrees Celsius and below. Special liquefied gases such as oxygen, nitrogen, and argon are required to reduce an object to such extreme temperatures. Other uses of such extreme temperatures include supercooling metals and liquids. For example, at 2.17K Helium becomes a superfluid and has zero viscosity. Though not very useful, this superfluid travels up the sides of a test tube and out the other side, defying laws of friction and gravity. Another example would be 4.2K niobium alloys used in most MRI scanners. When supercooled to this temperature, the metal provides no electrical resistance and requires no heat to keep it flowing. One final application could also be that of cryosurgery and its use of killing cancer by freezing the molecules beyond repair. Clearly cryogenics is a both fascinating and useful study.
The temperatures negative 150 degrees Celsius or 2.17K may not seem that low, but in actuality they are approaching the coldest temperature possible for any element or molecule. This point is called absolute zero. Absolute zero lies at negative 273.15 degrees Celsius or at 0K. These being the case, 2.17K or 4.2K are very close to the point of zero energy. Could this be the temperature at which a human body could remain frozen for thousands of years and emerge unscathed? Quite possibly, yes; but the energy required to do so would be near infinite. It would take an entire supernova to reach absolute zero for a brief moment, let alone thousands of years. The concept of a temperature where al energy has been removed is clearly revolutionary and could only have been discovered a short while ago. About 105 years ago to be more specific. In 1905 a German scientist by the name of Walter Nernst decided it would require infinite energy to reach such a point. He also supposedly discovered the point itself. How this is even possible is unfathomable, considering it would require so much energy to achieve such temperatures and conditions.
Delving into the details of temperature we come to German physicist Mr. Fahrenheit. In 1709 he invented one of the first modern style temperature measuring devices using alcohol. Like mercury it rose in capillaries with the addition of atmospheric heat. It wasn’t until 1714 that Fahrenheit created the mercury thermometer. This, apparently, was closer or more calibrated to the temperatures of Earth’s atmosphere than was alcohol. Later, in 1984, Theodore Hannes even went so far as to create a more practical thermometer that measured the body’s temperature through the ear. Thermometers had been used to measure body temperature for some time before that, but an ear thermometer was just more practical and much quicker. Curiously, like most other thermodynamic scientists, Hannes was from Germany. He even served as an air surgeon for the Luftwaffe during World War Two; perhaps his services as an air surgeon prompted his creation of a faster thermometer for the engaging in surgery.
William Thompson made a significant contribution to the scientific community with his addition to the thermometer: the Kelvin scale. How did a guy named William Thompson end up giving the name of Kelvin to his discovery? Excellent question, Thompson’s full name was, in fact, Lord William Thompson Kelvin 1st Baron. That is indeed quite a mouthful, but now you know how the name Kelvin came into the picture. In 1848, Kelvin created a scale that began at the lowest temperature possible, absolute zero, and climbed at the same rate as the scale created by Celsius. This is when the dates become confusing, because supposedly absolute zero was not discovered until 1905; perhaps Lord Kelvin was only making a theory, much like renowned scientist Albert Einstein. Unlike Walter Nernst, Lord Kelvin called this extreme temperature absolute temperature, rather than absolute zero. Thompson also created the second law of thermodynamics: that heat must flow from higher temperature to lower temperature unless there is the presence of an agent. Obviously it wasn’t a law yet, but Mr. Thompson was its inventor.
In the mid 1600’s another law relating to thermodynamics was discovered. This law is now called Boyle’s Law, presumably after a scientist with the name of Boyle. Boyle’s Law states that pressure and volume will be directly related before and after a reaction. Pressure may not be the same or volume may not be the same, but the combination of pressure and volume when multiplied would be the same before and after a physical reaction; provided the system is properly sealed. Think of a piston filled with gas, the pressure is 2 atm and the volume is 2L. As the piston comes down and compresses the gas, the volume halves to 1L while the pressure doubles to 4 atm. Before and after the reaction, pressure * volume = four. This law is always true because as particles are reduced to a smaller space and pushed closer together, the resistance and the desire to expand become proportionally stronger.
Charles’ Law also made a significant contribution to the study of thermodynamics. Obviously discovered by a man named Charles, this law emphasizes the relationship of volume and temperature in physical reactions. Charles states that volume and temperature are directly related both during experiments and outside the lab. He says that if temperature goes up by 50%, then volume must go up by 50% as well. This law makes perfect sense considering the molecules of a gas are constantly bouncing around with kinetic energy and expanding to fill their container. Increasing the temperature increases the energy and the force with which the molecules bounce and expand. The opposite is true when the temperature is reduced.
Returning to nuclear energy and thermodynamics we find scientists Stefan and Boltzmann. These two created a system that could be used to measure the amount of radiation created from heat in Kelvin’s. Radiation is simply energy given off in electromagnetic waves. Technically this means everything the human eye can see, the radio waves in the air, the microwaves in the kitchen, the X-rays at the dentist’s office and ultraviolet rays from the Sun can be measured for radiation energy. Boltzmann managed to put all these things into one unit of measurement.
The last two elements of thermodynamics being discussed here are very closely related. These elements are the Caloric Theory and the Carnot Engine. In Paris, Mr. Carnot wrote a document about how the street lights of France’s capital could be improved. This document was written in 1824 and was widely accepted. In this document Carnot discussed the Caloric Theory, which basically described an engine that could, ideally, put out as much work as was put in. This engine came to be called the Carnot engine, not because he created one, but because of his extensive research on the subject. An engine such as the Carnot engine could be created and would be very useful if humans lived for over a thousand years. As it stands, a Carnot engine may give out just as much work as is put in, but it would take so long to do so that it would simply be impractical.
Clearly the study of heat and its transformation into other forms of energy can be a fascinating pass time. Thermodynamics is affecting us every day and in everything humans do; it has been affecting mankind for many thousands of years. But only recently has this species gone on to explore it.
Works Cited
- http://www.grc.nasa.gov/WWW/K-12/airplane/aboyle.html
- http://www.scibooks.org/absolutezero.html
- http://inventors.about.com/library/inventors/blthermometer.htm
- http://library.thinkquest.org/12596/charles.html
- http://cti.itc.virginia.edu/~meg3c/classes/tcc313/200rprojs/lavoisier2/home.html
- http://iopscience.iop.org/1478-7814/23/1/315
- http://hyperphysics.phy-astr.gsu.edu/hbase/thermo/carnot.html
- http://apollo.lsc.vsc.edu/classes/met130/notes/chapter2/sb_law.html
- http://cryogenics.nist.gov/AboutCryogenics/about%20cryogenics.htm
Source : http://ehlersjo.files.wordpress.com/2010/08/physics-thermodynamics-essay1.doc
Web site link: http://ehlersjo.wordpress.com/
Google key word : Thermodynamics laws explained with examples for kids file type : doc
Author : J.Ehlers
If you are the author of the text above and you not agree to share your knowledge for teaching, research, scholarship (for fair use as indicated in the United States copyrigh low) please send us an e-mail and we will remove your text quickly.
Thermodynamics laws explained with examples for kids
Chapter 10: Thermodynamics
Up to this point in the course, only mechanical problems have been discussed. The quantities that describe mechanics can be expressed in terms of three fundamental quantities: mass, length, and time. This chapter discusses quantities that are expressed in terms of a fourth fundamental quantity: temperature.
A. Zeroth Law of Thermodynamics
[1] a. The Zeroth Law of Thermodynamics: Two substances at the same temperature are in thermal equilibrium since they cannot exchange heat with each other.
b. The significance of this law is the definitions of the terms temperature, heat, and thermal equilibrium.
[2] a. Temperature is a measure of the “hotness” of a substance. Temperature is a scalar quantity. The numerical specification of “hotness” must be based on a reliable and reproducible measurement.
b. All substances change state at a constant temperature. Initial temperature scales used the freezing point and the boiling point of water as a reference. For the Celsius scale, 0°C is defined as the temperature of melting ice at normal air pressure, and 100 °C is defined as the temperature of boiling water at normal air pressure.
c. -273.15°C is believed to be absolute zero, which is the lowest temperature a substance is able to achieve. The Kelvin scale was created based on this absolute zero, where the Kelvin scale is the Celsius scale shifted downward so as to keep temperatures above zero. Thus, a temperature measured on the Celsius scale is converted to the Kelvin scale (TK) as follows: TK = Tc + 273.15°C
Kelvin is the SI unit of temperature. A temperature measured in Kelvin is called the absolute temperature since the Kelvin scale is based on absolute zero. The modern standard for Kelvin is the triple point of water, which is defined to be 273.16 K at a pressure of 4.58 mm of mercury.
d. The above relation can be used to show that a one degree Celsius temperature change is equal to a one Kelvin temperature change. For example, when the temperature goes from 0°C to 21°C, the change in temperature is +21°C. Using the corresponding Kelvin temperatures, a temperature change from 273.15 K to 294.15 K gives a temperature change of +21 K. As such, degrees Celsius can be used interchangeably with Kelvin for a change in temperature.
e. Temperature also has a mathematical definition, which is that temperature is a measure of the kinetic energy of a molecule. The faster the molecule moves, the hotter its surroundings become, and thus the higher the temperature of the substance as a whole.
[3] a. Heat is defined as the energy that flows from a hot substance to a cold substance due only to their difference in temperature. Thus, two substances do not exchange heat when they are at the same temperature. Heat is also created from frictional effects.
b. Being hot does not mean that a hot substance contains more heat than a cold one. It just means that it is hotter. Furthermore, two substances that are equally hot do not in general have the same capacity to exchange heat. For example, some foods can be eaten straight out of the oven without a problem, while others would cause burns if eaten straight out of the oven.
c. Like all forms of energy, heat does not exist in physical reality. The physical observables in this part of the course are temperature and state of a substance. Heat exchanges are a mathematical tool for predicting changes in temperature and state when two substances of different temperatures come into thermal contact.
d. Heat was once defined as a physically real fluid that flowed from a hot substance to a cold substance. This fluid was measured in calories, where one calorie is equal to 4.186 J. The fluid theory was discarded when heat was shown to be a form of energy and not a caloric fluid. As such, calories will not be used in this course. Do note that one food Calorie is equal to 1000 heat calories.
[4] Thermal equilibrium is established when two substances are at the same temperature. When at the same temperature, two substances are as equally as hot, thus they do not exchange heat, thus they do not undergo any change in temperature or state, and thus they are in thermal equilibrium.
B. First Law of Thermodynamics
[1] a. The First Law of Thermodynamics: The change in the internal energy (ΔU) of a substance is equal to the heat exchanged by the substance minus the work done. As such, heat can be converted into work.
ΔU = Q – W |
DU is positive if the internal energy of the substance increases (negative if decreased), Q is positive if heat is absorbed by the substance (negative if released), and W is positive if work is done by the substance as it expands (negative if work done on the substance as it contracts).
b. The significance of this law is that heat is a form of energy. As such, energy is neither created nor destroyed but merely changes from one form to another. Heat, internal energy, and work are three such forms of energy.
[2] a. The internal energy of a substance is defined as the energy possessed by the molecules of that substance. To change the temperature or state requires that the internal energy changes.
b. A change in temperature results from a change in molecular translational kinetic energy. The more translational kinetic energy a molecule has, the faster it moves. And the faster the molecules are moving, the hotter the substance is as a whole.
c. A change in state results from the work done to break or build molecular bonds. Breaking molecular bonds requires energy to be absorbed by the molecules, while building molecular bonds requires energy to be released by the molecules.
[3] Work is force times parallel distance moved. Force divided by area is pressure, while distance moved times area is change in volume. If the pressure is constant, then work can be calculated as pressure times change in volume.
W = P ΔV |
Since gases readily expand, gases can do work. On the other hand, solids and liquids do not expand readily, and thus they do negligible work as a result of thermal expansion.
[4] a. The heat capacity (c) of a substance is a measurement of the heat associated with a change in temperature. Values for the heat capacity of various substances are given in the physical constants section at end of this chapter. The heat capacity equation is:
Q = c m ΔT |
b. The higher the heat capacity of a substance, the more heat it must absorb to undergo the same change in temperature as a substance with the same mass but lower heat capacity. Thus, a substance with a lower heat capacity changes temperature more rapidly when the same heat is exchanged.
c. Since solids and liquids cannot convert heat into work as a result of thermal expansion, they have only one heat capacity. On the other hand, gases can do work as a result of thermal expansion, and thus they have two heat capacities: one at constant volume (Cv) and one at constant pressure (Cp).
d. Q = ΔU when a solid changes temperature, when a liquid changes temperature, and when a gas changes temperature at constant volume. In these cases, the heat exchange causes a change in internal energy only.
e. Q = ΔU + W when a gas changes temperature at constant pressure. In this case, some of the heat is converted into work, which requires that more heat is exchanged to cause the same change in internal energy for a gas at constant pressure compared to the same gas at constant volume. This means that Cp>Cv.
f. The change in internal energy of a gas is always equal to the heat that would be absorbed at constant volume, regardless of if its volume is actually held constant.
[5] a. The latent heat (L) of a substance is a measurement of the heat associated with a change in state. Values for the latent heat of various substances are given in the physical constants section at end of this chapter. The latent heat equation is:
Q = ± L m |
The positive sign is used when changing from a solid to a liquid and when changing from a liquid to a gas (heat absorbed). The negative sign is used when changing from a gas to a liquid and from a liquid to a solid (heat released). Note that the heat absorbed to break a molecular bond is equal to the heat released when said molecular bond is built.
b. The latent heat of fusion is used for solid-liquid state changes, while the latent heat of vaporization is used for gas-liquid state changes. Note that the latent heat of vaporization is greater than the latent heat of fusion for any given substance. This is because more energy is associated with a change of liquid-gas molecular bonds than with a change of liquid-solid molecular bonds.
c. The temperature at which the substance undergoes its change in state does not alter the amount of heat needed to make that change in state. For example, ice melts at 0°C, and its latent heat of fusion is 335 J/g. Copper melts at 1083°C, and its latent heat of fusion is 207 J/g. Thus, more heat is required to melt 1 g of ice at 0°C than it does to melt 1 g of copper at 1083°C. Do note that this example does not take in account the heat needed to get the copper to 1083°C.
[6] When two substances at different temperatures come in thermal contact, heat flows from the hot substance to the cold substance until thermal equilibrium is achieved. The heat released by the hot substance must equal the heat absorbed by the cold substance. As such, the total heat exchanged by the two substances is zero.
Σ Q = 0 |
The heat released or absorbed by a substance is due to a change in either temperature or state or both, where a substance must undergo a change in temperature or state independently.
C. Second Law of Thermodynamics
[1] a. Clausius Statement of Second Law of Thermodynamics: No process is possible whose sole result is the transfer of heat from a cold substance to a hot substance.
b. Kelvin Statement of Second Law of Thermodynamics: No process is possible whose sole result is the complete conversion of heat into work.
c. The significance of this law is that heat flows naturally from a hot substance to a cold substance only. This results in the impossibility of completely changing energy from the form of heat into the form of work.
[2] a. A heat engine is a device that converts heat into other forms of energy such as electrical energy or work. A heat engine is an excellent demonstration of both the First and Second Laws of Thermodynamics.
b. For example, a thermoelectric converter can be used to make a simple heat engine. To do so, two metal plates are attached to either side of the thermoelectric converter (the thermoelectric converter being a series of semiconductor devices), and a fan equipped with an electric motor is attached to the leads of the thermoelectric converter. To operate, one metal plate is placed into hot water and the other into cold water. Heat will flow through the device from the hot water toward the cold water. Via the series of semiconductor cells, some of this heat is converted into electrical energy (that is an electric current is created). This electrical energy is converted by the motor of the fan into work that turns the blades of the fan. As such, heat flowing through the thermoelectric converter is converted into work. However, not all the heat will be converted into work. Some of the heat will flow through the thermoelectric converter to the cold water as evident by the fact that the temperature of cold water will increase (the hot water will decrease in temperature but at a much faster rate). Furthermore, some of the heat will be lost due to the work done against friction and due to the conduction and radiation of heat from the thermoelectric converter and the fan to the outside air. As such, the thermoelectric converter proves that heat can be converted into work (First Law) and that heat cannot be completely converted into work (Kelvin Statement of Second Law).
Note: How semiconductor cells create current and how an electric current can make a fan turn is not going to be discussed in this course.
[3] a. In general, the Second Law means that nature is spontaneous in one direction only. For example, a dead body decays into dust spontaneously, but dust does not form into a body spontaneously.
b. On the molecular level, molecules naturally move to more disordered motion. For example, a gas is placed on the left side of the container shown below. The container has insulating walls so that heat cannot leave or enter the container, and a partition separates the gas on the left with the vacuum on the right.
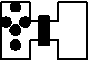
When the partition is taken away, the gas will expand to fill the entire container.

In doing so, no heat exchanges occurred because of the insulting walls, and no work was done because the gas had nothing to push against. However, the thermodynamic state of the gas has changed. This change is explained by the increase in disorder of the gas molecules as they expand to fill their container.
c. The entropy of a substance is a measure of the disorder of the molecules of that substance. All substances naturally move to increase entropy (toward more random motion). Entropy will not be calculated in this course.
D. Third Law of Thermodynamics
[1] a. The Third Law of Thermodynamics: As the temperature of a system tends to zero, the entropy of all parts of the system tends to zero.
b. The significance of this law is that the temperature of a substance decreases when order is produced in the system. At absolute zero, all parts of the system have to be perfectly ordered.
[2] a. The kinetic theory interpretation of temperature states that the temperature of a substance is a measure of the kinetic energy of a molecule of that substance. This is the classical interpretation.
b. The Third Law states that the temperature of a substance at low temperatures is a measure of the entropy (disorder) of the molecules of that substance. Thus, the kinetic theory does not apply at low temperatures.
c. At absolute zero, the molecules still have kinetic energy, as the molecules never stop moving. If they did stop, they would violate the Uncertainty Principle of Quantum Mechanics, as the exact speed and position of the molecule would be known simultaneously. This Uncertainty Principle will not be discussed further in this course. Suffice to say here that at absolute zero, the molecules are still moving but their motion would be completely ordered.
[3] Another consequence of the Third Law is that absolute zero can only be approached but not achieved because nature is not ideal. For example, figure (a) below shows an ideal crystal where the molecules of the substance show a definite, repeating order. Figure (b) shows how easily an imperfection can be made in a real crystal (and thus the entropy of all parts of the real crystal is not zero).

(a) (b)
Note: From the Zeroth Law of Thermodynamics, heat is exchanged by two substances at different temperatures in thermal contact. From the First Law of Thermodynamics, heat can be converted into work and is thus a form of energy. From the Second Law of Thermodynamics, heat is said to flow naturally from hot substances to cold substances only so that heat cannot be completely converted into work. From the Third Law of Thermodynamics, the temperature of a substance decreases at low temperatures when its molecules move to more ordered motion.
Chapter 10 Glossary
The Zeroth Law of Thermodynamics: Two substances at the same temperature are in thermal equilibrium since they cannot exchange heat with each other.
Temperature is a measure of the "hotness" of a substance.
Thermal equilibrium is established when two substances are at the same temperature.
Absolute zero is the lowest temperature that a substance is believed to be able to achieve which is – 273.15°C
A temperature measured in Kelvin is called the absolute temperature since the Kelvin scale is based on absolute zero.
Heat is the energy that flows from a hot substance to a cold substance due only to their difference in temperature.
The First Law of Thermodynamics: The change in the internal energy (DU) of a substance is equal to the heat exchanged by the substance minus the work done.
The internal energy of a substance is defined as the energy possessed by the molecules of that substance.
The heat capacity (c) of a substance is a measurement of the heat associated with a change in temperature.
The latent heat (L) of a substance is a measurement of the heat associated with a change in state.
Clausius Statement of Second Law of Thermodynamics: No process is possible whose sole result is the transfer of heat from a cold substance to a hot substance.
Kelvin Statement of Second Law of Thermodynamics: No process is possible whose sole result is the complete conversion of heat into work.
A heat engine is a device that converts heat into other forms of energy such as electrical energy or work.
The entropy of a substance is a measure of the disorder of the molecules of that substance.
The Third Law of Thermodynamics: As the temperature of a system tends to zero, the entropy of all parts of the system tends to zero.
Chapter 10 SI Units
Kelvin (K)
Chapter 10 Physical Constants
Substance |
heat capacity (in J/kg×°C) |
melting point (in °C) |
latent heat of fusion (in J/kg) |
boiling point (in °C) |
latent heat of vaporization (in J/kg) |
water |
4.186 x103 |
0 |
335 x 103 |
100 |
2260 x 103 |
copper |
.387 x 103 |
1083 |
207 x 103 |
2595 |
4730 x 103 |
lead |
.128 x 103 |
328 |
23.2 x 103 |
1750 |
870 x 103 |
aluminum |
.900 x 103 |
660 |
90.0 x 103 |
2450 |
11400 x 103 |
mercury |
.140 x 103 |
-39 |
11.4 x 103 |
357 |
296 x 103 |
germanium |
.322 x 103 |
------ |
------ |
------ |
------ |
gas |
heat capacity at constant volume (in J/kg×°C) |
heat capacity at constant pressure (in J/kg×°C) |
helium |
3.12 x 103 |
5.19 x 103 |
nitrogen |
.739 x 103 |
1.04 x 103 |
oxygen |
.650 x 103 |
.909 x 103 |
Chapter 10 Equations
Tk = Tc + 273.15 °C
ΔU = Q – W
W = P ΔV
Q = c m ΔT
Q = ± L m
Σ Q = 0
Chapter 10 Questions
[1] State what happens when ice at 0°C is dropped into water at 0°C.
[2] State the change undergone if (a) ice at 0°C absorbs heat and (b) ice at 0°C releases heat.
[3] State the change undergone if (a) steam at 100°C absorbs heat and (b) steam at 100°C releases heat.
[4] State the change undergone if (a) water at 80°C absorbs heat and (b) water at 80°C releases heat.
[5] Explain why water does not change temperature as quickly as most substances.
[6] Explain why more damage is done to one's hand when it is placed in 100°C steam instead of 100°C water.
[7] Explain why condensation is a warming process and why evaporation is a cooling process.
[8] Explain the basic meanings of the four laws of Thermodynamics.
[9] Explain why an increase in internal energy and not the absorption of heat energy determines the magnitude of the increase in temperature of a gas.
[10] Explain why an absorption of one Joule of heat energy equals a one Joule increase in internal energy in a solid or a liquid when changing temperature but not necessarily so for a gas that is changing temperature.
[11] Explain why gases have two specific heats while solids and liquids each have one.
[12] Explain how the temperature of a substance is decreased when near absolute zero and when near normal temperatures.
[13] Explain why the molecules of a substance are still moving at absolute zero.
conservation of heat
In all the remaining problems, ignore heat exchanges with the surroundings and with the container unless otherwise specified.
Source : http://www.dwmblog.com/abtech/spr2012/phy151/Text/10-Thermo.doc
Web site link: http://www.dwmblog.com/abtech/
Google key word : Thermodynamics laws explained with examples for kids file type : doc
Author : not indicated on the source document of the above text
If you are the author of the text above and you not agree to share your knowledge for teaching, research, scholarship (for fair use as indicated in the United States copyrigh low) please send us an e-mail and we will remove your text quickly.
Thermodynamics laws explained with examples for kids
If you want to quickly find the pages about a particular topic as Thermodynamics laws explained with examples for kids use the following search engine:
Thermodynamics laws explained with examples for kids
Please visit our home page
Larapedia.com Terms of service and privacy page